3212
Fast self-gated 4D flow measurements in the murine aortic arch with retrospective radial sampling1Experimentelle Physik V, Universität Würzburg, Würzburg, Germany, 2Medizinische Klinik und Poliklinik I des Universitätsklinikums Würzburg, Würzburg, Germany, 3Universitätsklinikum Würzburg, Institut für Diagnostische und Interventionelle Radiologie, Würzburg, Germany, 4Fraunhofer IIS, Fraunhofer EZRT, Magnetresonanz- und Röntgenbildgebung (MRB), Universität Würzburg, Würzburg, Germany
Synopsis
A self-navigated radial 4D-PC sequence is presented for accelerated ECG-free 4D flow measurements in the murine aortic arch. Self-navigation signals were extracted from the radial DC signal and used for retrospective motion synchronization. 3D-Cines with 30 frames were reconstructed with a spatial resolution of 100 µm. The volume flow was determined at 4 2D slices extracted from the 3D dataset and the 3D flow was visualized with streamlines. The results are in good accordance with results reported for ECG-triggered measurements. The new method yields high potential for preclinical studies of hemodynamics and can also be transferred to applications in humans.
Purpose
Flow quantification using 4D PC-Cine MRI became recently an important tool for preclinical studies of atherosclerosis, which enables non-invasive measurements of cardiovascular hemodynamics as well as the assessment of functional parameters such as the aortic compliance and endothelial shear stress. Major disadvantages of previously introduced triggered 4D flow quantification techniques are the very long measurement time needed to achieve the necessary high temporal and spatial resolutions and the dependency on stable cardiac motion synchronization signals. Especially in small rodents flow measurements are challenging due to the difficult animal handling and the ECG signal’s vulnerability to errors at high magnetic field strengths. In this abstract we present a new self-navigated 4D PC-Cine MRI technique that enables high-resolution (100 µm isotropic) 4D flow measurements of the complete murine aortic arch in 32 minutes. The use of retrospective reconstruction and self-gating enables a very flexible data analysis and increases the robustness since an ECG signal is no longer needed for cardiac motion synchronization.Materials and Methods
MR measurements
All measurements are carried out on a 17.6T small animal MRI scanner with a 1 T/m gradient system and a 24 mm birdcage coil. Mice were anesthetized with isoflurane (1.5-2% in oxygen) and kept on a constant body temperature of 37°C. For the 3-dimensional flow encoding a radial 4D-PC-MRI sequence with a balanced 4-point encoding sequence1 was used. The acquisition parameters were: VENC=125 cm/s, TR/TE=3.0/1.1 ms, echo asymmetry: 10%, 160000 spokes, FOV: 25x25x4 mm3. For slice excitation a sinc-shaped pulse with a flip angle of 15° was used. The 4D flow measurements were conducted without triggering during free breathing and required a total scan time of 32 minutes.
Reconstruction
Cardiac and respiratory motion signals were extracted from the radial DC signal. High frequency noise was suppressed with a matched filter2 and a baseline correction was applied. The processed self-gating signals were afterwards used for breath gating and for the assessment of trigger time stamps and the relative positions in the heart cycle, as described recently3,4. The information about the cardiac phases was used for the subsequent sliding window (window width: 1/30 of the cardiac period) selection of the radial projections needed for the 4D-Cine reconstruction. For each velocity encoding step 3D images with an isotropic spatial resolution of 100x100x100 µm3 were reconstructed with convolution gridding5 at 30 different cardiac phases. All reconstructions were done with Matlab (The Mathworks, Inc., Natick, USA) and the velocity information was visualized with Ensight (CEI Software, USA).
Results
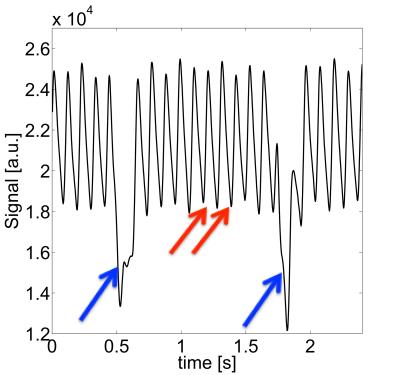
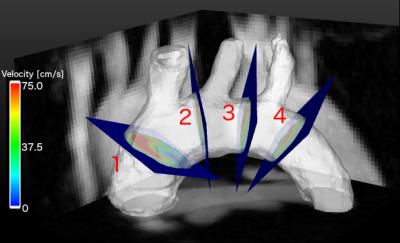
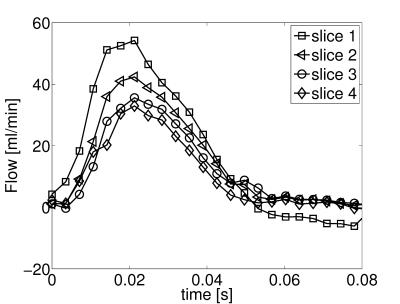
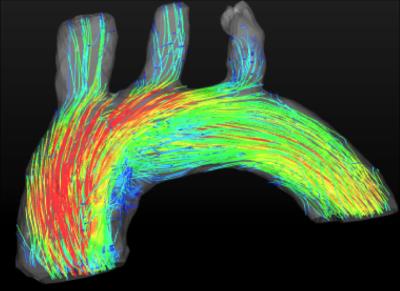
Self-navigated 4D flow measurements were conducted in the murine aortic arch. Fig. 1 shows an example of a self-gating signal measured in an ApoE-/- mouse. The modulations due to cardiac motion (red arrows) and respiration (blue arrows) are clearly recognizable. The mean cardiac period during this measurement was (106.4±3.4) ms. Fig. 2 illustrates a 3D isosurface of the arch at the systolic cardiac phase together with maximum intensity projections of the magnitude image. Four slices were extracted from the 3D dataset to determine the volume flow through the aorta. The results can be seen in Fig. 3 (from slice 1: ascending aorta to slice 4: descending aorta). The peak flow decreases for slices with larger distances to the aortic root due to the partial blood flow into the major branches. The flow values are in good accordance with previous studies6. Fig. 4 shows a streamline illustration of the blood flow through the aortic arch and the smaller arteries.![]() Discussion and Conclusion
Discussion and Conclusion
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 688 B5, Z2), the Bundesministerium für Bildung und Forschung (BMBF01 EO1004) and the Comprehensive Heart Failure Center (CHFC).References
[1] Pelc et al., Jour Magn Reson Med, [1991]; 405-413
[2] Herold et al., Proc. ISMRM 2016; 0466
[3] Winter et al., JCMR, [2013], 15:88-98
[4] Winter et al., Magn Reson Med, [2016]; DOI: 10.1002/mrm.26068
[5] Fessler JA; IEEE Trans Signal Process 2003;51(2):560–574.
[6] Janizcek et al., Magn Reson Med, [2011]; 66:1382-1390
Figures