3209
Variable Density CAIPIRINHA for Highly Accelerated Volumetric Dynamic Contrast-Enhanced Imaging of Liver1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, People's Republic of China, 2Harvard-MIT Health Sciences and Technology, MIT, Cambridge, MA, United States, 3Department of Radiology, Stanford University, Stanford, CA, United States, 4Department of Electrical Engineering, Stanford University, Stanford, CA, United States, 5Neusoft Medical System, People's Republic of China
Synopsis
Dynamic Contrast-Enhanced (DCE) imaging is widely used in detection and characterization of many liver diseases. In clinical practice, breath holding is commonly used to overcome respiratory motion and get high quality images. However, long breath holds can be painful or unavailable for some patients. To address this problem, we present a variable density 2D CAIPIRINHA sampling technique with a novel reconstruction framework for highly accelerated volumetric DCE liver imaging. Simulation and in-vivo experiments were performed to evaluate the performance of the proposed method compared with other current techniques. The results show great improvement of image quality within shorter acquisition time.
Purpose
Dynamic Contrast-Enhanced (DCE) imaging is widely used in detection and characterization of many liver diseases. But sometimes it is challenging to get high quality DCE images in presence of respiratory motion during acquisition. Therefore, images are often acquired within several breath holds in clinical practice. However, long breath holds can be painful or unavailable for some patients. In order to address this problem, fast imaging techniques are required to get high quality images with less acquisition time. Among these techniques, the Controlled Aliasing in Volumetric Parallel Imaging (2D CAIPIRINHA) 1 has been demonstrated to be capable of reducing g-factor of parallel imaging and thus improving image quality of volumetric imaging even at high acceleration factors. In this study, we present a variable density (VD) CAIPIRINHA sampling technique with a novel reconstruction framework for highly accelerated volumetric imaging, and validate its application on DCE liver imaging. The proposed method uses the 4D k-space data more efficiently, thus the improved image quality and reduced acquisition time (breath holding time) with high undersampling factors can be achieved.Methods
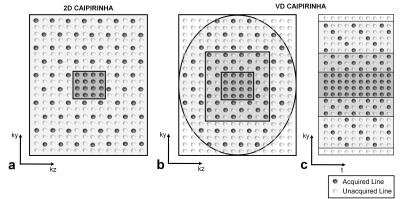
The variable density (VD) CAIPIRINHA is proposed to reduce aliasing artifacts by sampling the center of k-space with a higher rate, and to reduce acquisition time by sampling the outer region of k-space with a lower rate. As shown in Figure 1, 3D k-space data are divided into four parts (center square, middle square, outer ellipse and outer square) according to their distance to the k-space center. Each part is sampled using 2D CAIPIRINHA pattern with a different acceleration factor. The shifts are also applied in time dimension to make full use of data (Figure 1c).
A novel reconstruction framework is developed for this sampling pattern. 4D k-t data sets are used in the reconstruction process. The fully sampled data in the center square of k-space is first used as auto-calibration signal (ACS) to reconstruct the middle square of k-space for each time frame using a parallel imaging method, SPIRiT constrained by data fidelity and data consistency 2. Then, the center square and reconstructed middle square of k-space are served as ACS along all time frames, to reconstruct the whole 4D k-space data using k-t interpolation.
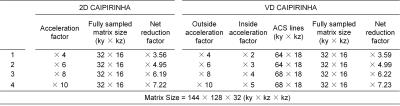
Simulations: First simulation study was designed to test the performance of the proposed sampling method at various acceleration factors compared with the conventional 2D CAIPIRINHA. Fully-sampled data were obtained on a healthy volunteer on a 3T Phillips scanner (Philips, Best, the Netherlands) and then were retrospectively undersampled with acceleration factors as shown in Table 1 using 2D CAIPIRINHA and VD CAIPIRINHA. Second simulation was performed to evaluate the proposed method on DCE images. The images were reconstructed using the proposed reconstruction framework for both sampling patterns at acceleration factors of 7.08 and 7.83.
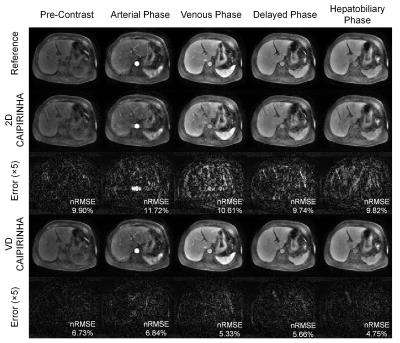
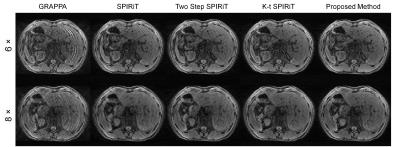
In-vivo Experiments: In-vivo Experiments were performed to validate the ability of the proposed method to get relatively high quality images at high acceleration factors. Perspective undersampled data with VD CAIPIRINHA pattern were acquired on a healthy volunteer with breath holding using 3D-TFE on the 3T Phillips scanner at acceleration factors of 6 and 8 respectively. The acquisition parameters were: TR/TE = 2.8/1.34 ms, matrix size= 128×144×32, FOV = 256×288×128 mm3. Different reconstruction methods (GRAPPA 3, SPIRiT 2, k-t SPIRiT 4,5) were implemented to compare with the proposed reconstruction framework.
Results
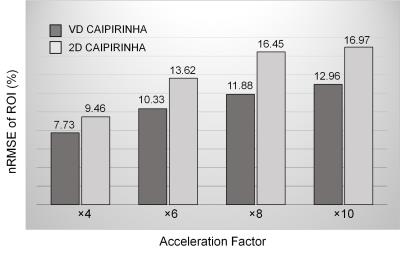
Figure 2 shows normalized root-mean-square errors (nRMSEs) of 3D reconstructed images from undersampled dataset using VD CAIPIRINHA and 2D CAIPIRINHA at different acceleration factors (net reduction factors are listed in Table 1). VD CAIPIRINHA results in lower nRMSEs than the conventional 2D CAIPIRINHA at all acceleration factors tested. Figure 3 shows reconstructed DCE images and corresponding error maps at pre-contrast, arterial phase, venous phase, delayed phase and hepatobiliary phase of a single slice, using fully-sampled images as the reference. The proposed VD CAIPIRINHA significantly reduced the aliasing artifacts due to undersampling and resulted in better image quality. The results of in-vivo experiment are shown in Figure 4. The proposed reconstruction framework performed better than other methods.Discussion and Conclusion
A novel sampling strategy with a reconstruction framework is proposed for DCE liver imaging to get high quality images with shorter acquisition time (2~3s/volume of in-vivo experiment), thus to ease patients’ pain of long breath holds. Two simulation studies validate its ability to reduce aliasing artifacts at high acceleration factors and its effectiveness for DCE imaging with improved accuracy respectively. The in-vivo experiments preliminarily demonstrate the feasibility of the proposed sampling method and the reconstruction framework with perspective sampling data.Acknowledgements
This work is supported by NSFC funding 81371540, 81571667; and Philips funding.References
1. Breuer FA, Blaimer M, Mueller MF, Seiberlich N, Heidemann RM, Griswold MA, Jakob PM. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magnetic resonance in medicine 2006;55(3):549-556.
2. Lustig M, Pauly JM. SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space. Magnetic Resonance in Medicine 2010;64(2):457-471.
3. Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magnetic resonance in medicine 2002;47(6):1202-1210.
4. Lai P, Lustig M, Brau AC, Vasanawala S. kt SPIRiT for ultra-fast cardiac cine imaging with prospective or retrospective cardiac gating. In Proc Intl Soc Mag Reson Med, 2010. p 482.
5. Santelli C, Schaeffter T, Kozerke S. Radial k-t SPIRiT: Autocalibrated parallel imaging for generalized phase-contrast MRI. Magnetic resonance in medicine 2014;72(5):1233-1245.
Figures