3207
Using MRI to assess alterations in liver blood flow and oxygenation in response to physiological stress tests: meal challenge, hypercapnia and hyperoxia.1Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 2NIHR Nottingham Digestive Diseases Biomedical Research Unit, Nottingham University Hospitals Trust and the University of Nottingham, Nottingham, United Kingdom
Synopsis
Assessment of the capacity for dynamic changes in liver blood flow and oxygenation may provide a mechanism to improve the stratification of chronic liver injury. Here we assess dynamic hepatic blood flow and liver T2* alterations in response to postprandial hyperaemia following a meal, and hypercapnia and hyperoxia gas challenges. We show significant changes in blood flow and T2* in response to these challenges, and highlight that both the mode and FWHM of the T2* distribution should be assessed. However, such stress tests can only be applied in participants with higher baseline T2* for any change to be evident.
Purpose
Liver disease is one of the five most common causes of death in the UK and its prevalence is increasing. Liver fibrosis and cirrhosis are asymptomatic until the latter stages of disease and the current tools to stratify liver disease either lack sensitivity and specificity (e.g. liver function tests, ultrasound) or are invasive (liver biopsy). The assessment of the capacity for dynamic changes in liver blood flow may provide a mechanism by which to improve the stratification of chronic liver injury.
AIM: To assess dynamic hepatic blood flow and liver T2* alterations in response to postprandial hyperaemia following a mixed meal, and hypercapnia and hyperoxia gas challenges.
Methods
10 healthy participants (5M/5F, age 22-56y) attended two scan sessions following an overnight fast and completed the following stress challenges:
MEAL CHALLENGE: Baseline data (3 repeats) were acquired prior to ingestion of a standard meal (440ml Ensure plus, 660kcal, 22g fat, 89g carb, 28g protein)1 and subjects were scanned again after 20 mins.
GAS CHALLENGE: A sequential gas delivery breathing circuit and a prospective, feed-forward gas delivery system (Respiract™, Thornhill Research Inc., Canada) was used to control and monitor end-tidal O2 (PETO2) and CO2 (PETCO2) partial pressures. Normoxia was targeted at the subject’s resting value (PETO2 ~100mmHg) and hyperoxia at PETO2 ~500mmHg. Isocapnia was maintained at the subject’s resting value (PETCO2 ~40mmHg) with hypercapnia aimed at PETCO2 ~6mmHg above. The paradigm consisted of 5 blocks. Blocks 1, 3 and 5: 5 min at resting PETCO2 and PETO2. Blocks 2 and 4: 5 mins of hyperoxia or hypercapnia (random order).
Imaging was performed on a 3T Philips Achieva scanner (SENSE XL torso coil). Blood Flow: Phase contrast MRI2 was used to assess portal vein (PV) and hepatic artery (HA) flow, placing an imaging slice perpendicular to each vessel (FA 25°, reconstructed voxels 1.17x1.17x6mm3, ~15s breath hold, PV: Phases 20, TR/TE 8.4/3.7ms, VENC 50cm/s, HA: Phases 30, TR/TE 5.6/3.2ms, VENC 100 cm/s). T2*: A multi-echo fast field echo (mFFE) sequence was used with 8 contiguous axial slices (1x1x8mm3, SENSE 2, TE/ΔTE 2.5/2.5ms, 12 echoes, FA 30°, ~17s breath hold).
Data Analysis
Q-flow software (Philips) was used to estimate PV and HA velocity and flux. T2* maps were formed by fitting to an exponential signal decay. A region of interest was drawn over the liver on each slice. Histogram analysis was used to assess the mode and full-width-at-half-maximum (FWHM) of the T2* distribution across the liver. Baseline data was averaged across three measures for both the meal and gas challenge, and the coefficient of variation (CoV) across challenges calculated for each subject. For each challenge, the change from associated baseline was calculated.
Results
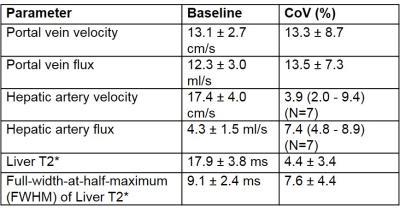
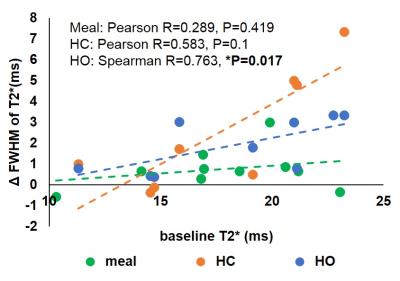
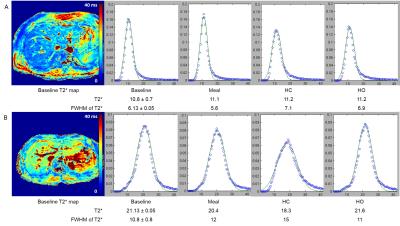
Figure 1 provides baseline data for PV and HA blood flow and liver T2*. The CoV is highest for the PV, with flux having a higher CoV than velocity for both vessels, due to errors associated with area measurement. HA flow and liver T2* had similar CoVs. Figure 2A-D shows the change from baseline in blood flow for each challenge. PV velocity increases significantly after the meal and on hypercapnia, whilst PV flux only increases after the meal. Following the meal, HA velocity shows a trend for reduction, whilst HA flux significantly decreases. Figure 2E-F shows the change from baseline of the mode and FWHM of liver T2*. There was a trend for T2* to increase after the meal and for hyperoxia, with a decrease on hypercapnia, whilst the FWHM of T2* significantly increased after all challenges. Figure 3 shows the change in T2* FWHM against baseline T2* for each challenge, demonstrating that participants with lower baseline T2* have less capacity for change following any stress test. This is highlighted in Figure 4 where example T2* maps and histograms are shown for subjects with short (11 ms) and longer (21 ms) baseline T2* for each stress test, illustrating the broader FWHM in subjects with higher baseline T2*.Discussion
Here we demonstrate the use of liver stress tests following a meal, and hypercapnia and hyperoxia gas challenges. Results of the gas challenge are in agreement with the findings in the rat model3,4: hypercapnia increased PV velocity and decreased HA velocity, leading to reduced liver T2* due to higher deoxyhaemoglobin levels associated with deoxygenated PV blood flow; hyperoxia increased liver T2* due to the high liver blood content and hypervascularity and low baseline oxyhaemoglobin level. This increase in liver T2* has been seen in healthy humans.5,6 The meal induced an expected large increase in PV flow, this resulted in increased liver T2* as oxygen consumption decreased.7Acknowledgements
Financial support from the MRC Confidence in Concept Award and NIHR Nottingham Digestive Diseases Biomedical Research Unit, Nottingham University Hospitals NHS Trust and University of Nottingham.References
1. Albillos, A et al., Gut. 2007; 56(2): 259-64.
2. Debatin, JF, Abdom. Im. 1998; 23: 485-95.
3. Barrash, H et al., Radiology. 2007; 243(3): 727-735.
4. Ganesh, T et al., J. Magn. Reson. Imaging. 2016; 44:305-316.
5. Patterson, AJ et al., J. Magn. Reson. Imaging. 2016; 44:739-744.
6. O’Connor, JPB et al., Magn. Reson. Med., 2009; 61:75-83.
7. Haque, M et al., J. Magn. Reson. Imaging. 2010; 32:988-991.
Figures