3201
Weighted k-t SPIRiT with Golden Angle Radial Sampling for Dynamic Contrast-Enhanced Liver Imaging1Department of Biomedical Engineering, Beijing Jiaotong University, Beijing, People's Republic of China, 2Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, People's Republic of China, 3Key Laboratory of Particle and Radiation Imaging, Ministry of Education, Medical Engineering and Institute, Department of Engineering Physics, Tsinghua University, Beijing, People's Republic of China
Synopsis
Acquiring high spatial-temporal resolution images is important for dynamic contrast-enhanced (DCE) imaging. In this work, we proposed a weighted k-t SPIRiT method and tested it in both simulation and in-vivo liver imaging studies based on motion-insensitive golden angle radial stack-of-stars sampling. In this study, feasibility of the weighted k-t SPIRiT has been validated. The results showed that, weighted k-t SPIRiT can improve the image quality compared with SPIRiT, while preserve highly accurate temporal information.
Target Audience
Radiologists and scientists working on hepatic DCE imaging.Purpose
Measuring the tissue’s enhancement in dynamic contrast-enhanced (DCE) is important in clinic. However, high spatial-temporal resolution in dynamic imaging is desired but challenged to achieve. In recent years, reconstruction methods have been proposed to pursue high spatial-temporal resolution with high under-sample rate, such as the SPIRiT1. Recently, k-t SPIRiT2 was proposed to further improve the image quality under high under-sample factors by further utilizing the abundant information in temporal dimension. However, the temporal information, which is very important in diagnosis, will be distorted in k-t SPIRiT. Thus, this study aims to propose a weighted k-t SPIRiT method to achieve good image quality while preserve the important temporal information in dynamic imaging.
Methods
Theory: In the proposed method, the k-space data from several adjacent time frames are used in reconstruction by solving the following optimization problem based on SPIRiT:
$$$\quad \quad \quad \quad\quad\quad\quad \quad \quad\quad \quad \quad \quad argmin(d_{c})\quad \parallel W_{i}d_{r}-Dd_{c}\parallel_2^2+\lambda^{2}\parallel(G-I)d_{c}\parallel_2^2$$$
where dc is the reconstructed Cartesian k-space data of all channels at current time frame, dr is the acquired radial k-space data, D represents a gridding matrix for non-Cartesian sampling which also selects the acquired k-space points out of dc, G is the SPIRiT interpolation operator, I is the identity matrix, λ is the regularization parameter to adjust data consistency and calibration consistency. Different from SPIRiT and k-t SPIRiT, a temporal weighted factor Wi of dr at the ith iteration is added to better preserve the temporal information.
Experiments:
Simulation tests: An in-vivo acquired non-contrast T1-weighted liver image was used in simulation. First, the regions of interests (ROIs) for aorta, portal vein, and liver tissue were outlined by hand. Then the DCE images were simulated by using simulated arterial input function (AIF) curve, portal venous input function (PIF) curve and liver tissue curves. AIF and PIF were generated by an empirical equation3 fitted by a set of human data4, and were applied to the aorta and portal vein ROIs, respectively. The intensity change in liver tissue was generated by Van Beers model5 with model parameter Fa=21.0 mL/100g/min, Fp=76.2 mL/100g/min, and Ko=0.1085/s. Then 3600 spokes were generated using golden angle radial stack-of-stars trajectory6 to simulate 360s DCE acquisition.
In-vivo experiments: A healthy volunteer was scanned using a 36-channel torso cardiac coil with a golden angle stack-of-stars sampling DCE sequence on a 3.0T Phillips scanner (Achieva, TX, Philips) with following parameters: FOV=320×320×200 mm³, TR/TE=3.60/1.44ms, FA 20°, totally 360s axial data were acquired (1280 projections per slice, 256 readout points per projection), in-plane resolution 1.25×1.25mm, slice thickness 5mm, 51 slices with fat suppression. Simultaneous to the beginning of the scan, 0.1mmol/kg Gd-DTPA was injected by a power injector at 2ml/s followed by a saline flush.
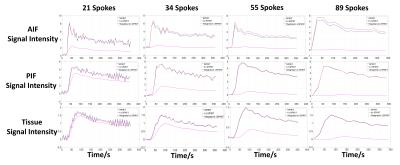
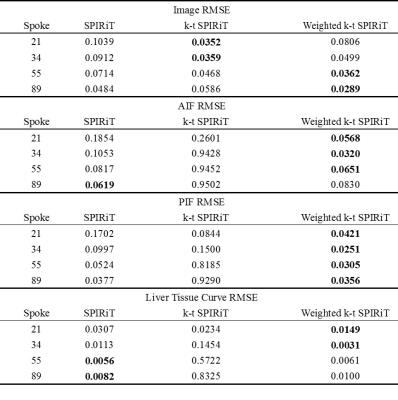
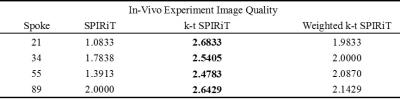
Image reconstruction and analysis: Dynamic images were reconstructed with 21, 34, 55 and 89 spokes per frame respectively for both simulated and in-vivo k-space data using SPIRiT, k-t SPIRiT and weighted k-t SPIRiT. In simulation, the root-mean-square error (RMSE) of both reconstructed images and curves (AIF, PIF, liver tissue) were calculated to evaluate the accuracy of reconstruction. For in-vivo experiments, image qualities were rated by 2 volunteers from 1(lowest) to 3(highest) in a blind way, and the AIF, PIF and liver tissue curves were also reported.
Results
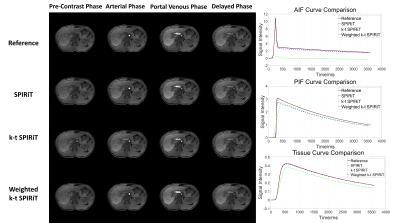
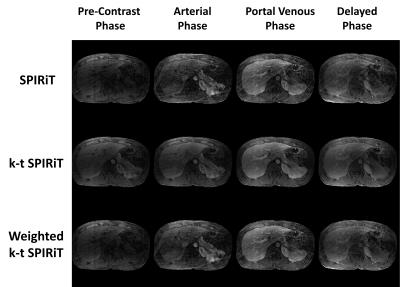
In simulation tests, compared with SPIRiT, improved image quality (Fig 1) and smaller RMSE (Table 1) can be seen in k-t SPIRiT and weighted k-t SPIRiT. As for the intensity curves, k-t SPIRiT reconstruction distorted the signal curves severely, especially for fast changing AIF (Fig 1). On the other hand, weighted k-t SPIRiT and SPIRiT have less distortion (Fig 1) and much smaller RMSE in the measurement of AIF, PIF and liver tissue curve (Table 1). In in-vivo experiments, the proposed weighted k-t SPIRiT method has the better image quality than SPIRiT, but not as high as k-t SPIRiT (Table 2 and Fig 2). However, k-t SPIRiT has much lower enhancement in AIF, PIF and liver tissue curve than weighted k-t SPIRiT and SPIRiT (Fig 3). The proposed weighted k-t SPIRiT has similar signal curves with SPIRiT.
Discussion and Conclusion
The results of this study showed that the proposed weighted k-t SPIRiT improves the image quality compared with SPIRiT in both simulation and in-vivo studies. Compared with k-t SPIRiT, weighted k-t SPIRiT has less temporal distortion on the AIF, PIF, and liver tissue curves, whose accuracy are important in the analysis of DCE images. In conclusion, the proposed weighted k-t SPIRiT is a good method to achieve high spatial-temporal resolution while keeping accurate temporal information.
Acknowledgements
This work is supported by NSFC funding 81571667 and 81371540.
References
1. Lustig, Michael, and John M. Pauly. "SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space." Magnetic resonance in medicine 64.2 (2010): 457-471.
2. Lai, P., et al. "kt SPIRiT for ultra-fast cardiac cine imaging with prospective or retrospective cardiac gating." Proceedings of the 18th Annual Meeting of ISMRM, Stockholm, Sweden. 2010.
3. Li, Xia, et al. "A novel AIF tracking method and comparison of DCE-MRI parameters using individual and population-based AIFs in human breast cancer." Physics in medicine and biology 56.17 (2011): 5753.
4. Ning, Jia, et al. "Hepatic function imaging using dynamic Gd-EOB-DTPA enhanced MRI and pharmacokinetic modeling." Magnetic Resonance in Medicine (2016).
5. Materne, R., et al. "Assessment of hepatic perfusion parameters with dynamic MRI." Magnetic resonance in medicine 47.1 (2002): 135-142.
6. Feng, Li, et al. "Golden-angle radial sparse parallel MRI:
Combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible
dynamic volumetric MRI." Magnetic resonance in medicine 72.3 (2014):
707-717.
Figures