3184
Towards development of tools for quantitative body DWI1AIM Medical Imaging, Vancouver, BC, Canada
Synopsis
Body DWI is a technique that is potentially quantifiable, and numerous clinical publications have demonstrated tissue variability of the ADC parameter. The variability of machine hardware and acquisition parameters impacts the signal from which ADC is calculated. To address the needs of standardization of machines, a body diffusion phantom and analysis software is being developed. Preliminary results are presented here.
Background
Numerous clinical publications have demonstrated tissue variability of the apparent diffusion coefficient (ADC) parameter assessed using body diffusion weighted imaging (DWI) technique. Diffusion weighted imaging in the body is a MRI technique that relies upon tracking bulk movement of water within a local tissue microenvironment. This technique has the capability of being quantifiable, with the slope representing the signal intensity between multiple different diffusion gradient pulses (b-values). This slope results in the metric of apparent diffusion coefficient (ADC). Considerable effort is underway to make b-values and ADC parameters machine independent and reproducible with the ultimate goal of developing an imaging biomarker from this technique which should relate to the degree of water tortuosity and thus represent the cellularity and nuclear cytoplasmic ratio of specific tissue.
The signal intensity of MRI machines for specific b-values is dependent on gradient field strength, coil sensitivity, shimming, image signal processing as well as imaging protocols. The effect of these hardware and software parameters on signal-to-noise and ADC values is therefore variable. The effect of these parameters in head coils is being studied with a NIST developed head phantom with the Quantitative Imaging Biomarker Alliance (QIBA) of the RSNA1 and the IMI QuIC-ConCepT of the EORTC2.
Body diffusion weighted imaging is more susceptible to differences in signal-to-noise and ADC values secondary to variation in body habitus, coil placement, motion (breathing etc.), specialized body signal processing and sequence selection. Thus in order to determine how these parameters effect signal-to-noise and ultimately ADC, steps towards development of a body diffusion phantom and analysis tools are underway.Materials and Methods
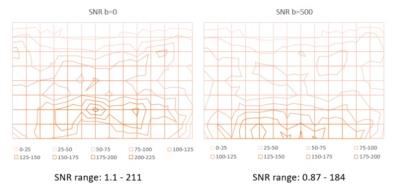
A homogeneous concentration of 37.5% polyvinylpyrrolidone was placed in a 40x20x9cm container and was scanned in multiple orientations on a 1.5 T Siemens Espree Tclass machine that has been optimized for whole body diffusion, using multiple different body matrix and spine coils at isocenter with b-values of 0 and 500. To assess phantom homogeneity the container was first placed sideways and scanned then flipped on the other side and rescanned. Signal to noise calculation profiles were obtained by 6 different acquisition passes and a 9x14 grid of 10mm diameter regions of interest were placed in the center of the axial imaged slab to calculate the signal to noise using QIBA software (figure 1).Results
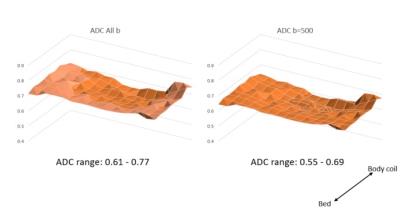
Signal to noise profiles in axial plane were obtained at b=0 and b=500 (figure 2). The range of signal variability was less at the higher b value (b=0 represents the maximal signal). Turning the container did not have effect on signal to noise ratio profiles. The impact of the signal to noise profile on ADC values at longer TR=15000ms and TE=107ms is shown in figure 3. The ADC values were higher close the bed (integrated spine coil) compared with the body coil. When using TR=5300ms and TE=67ms which are more typical of clinical scan ranges changes in ADC profile compared with longer TR and TE were identified (figure 4).Discussion and Conclusion
A homogeneous phantom permits evaluation of the effects of hardware and scan parameters. The findings demonstrate the impact of coil proximity and differences in sensitivity which impact the calculations of ADC. Differences in scan parameters also result in different axial profiles. Thus a phantom for hardware characterization and signal-to-noise optimization is critical to attempt to build standards for body DWI, as the impact of variable physiologic fat and gas interfaces also will likely effect signal-to-noise and thus ADC. Ongoing phantom and signal measurement software development will allow further characterization of body DWI with the goal of developing a reproducible metric for tissue characterization.Acknowledgements
We would like to thank Dr. Michael Boss of NIST for help with phantom development, and Dr. Tom Chenevert for development of ADC Signal to Noise software analysis tools.References
1. www.rsna.org/QIBA-Profiles-and-Protocols
2. www.quic-concept.eu
Figures