3179
Mean Kurtosis discriminates between low- and high-risk prostate cancer better than Mean diffusivity does1SAIMLAL Dept., Morphogenesis and Tissue Engineering, Sapienza University of Rome, Rome, Italy, 2Physics Dept., CNR ISC UOS Roma Sapienza, Rome, Italy, 3SAIMLAL Dept., Morpho-functional Sciences, Sapienza University of Rome, Rome, 4Department of Diagnostic and Interventional Radiology, Molecular Imaging and Radiotherapy, PTV Foundation, “Tor Vergata” University of Rome, Rome, Italy, 5Urology Unit, Department of Experimental Medicine and Surgery, 6Anatomic Pathology, Department of Biomedicine and Prevention, PTV Foundation, “Tor Vergata” University of Rome, 7Urology Unit, Department of Experimental Medicine and Surgery, PTV Foundation, “Tor Vergata” University of Rome, 8CNR ISC UOS Roma Sapienza, Rome, Italy
Synopsis
This work was finalized to compare the diagnostic potential of Diffusion Tensor and Diffusion Kurtosis Imaging in discriminating between low- and high-risk prostate cancer (Pca). Maps of Mean Diffusivity (MD), apparent Kurtosis (K) and apparent diffusion coefficient (D) were obtained from DWIs of 24 patients with different tumour grade. K maps better highlight differences between periferal PCa, PCa and benign tissue. In particular K discriminates between low- and high-risk PCa with a higher statistical significance compared to that of MD. DKI can improve the accuracy of the current PCa diagnosis providing a useful tool for PCa detection and grading.
Purpose
Prostate cancer (PCa) is the second most common malignancy and the fifth leading cause of death in men worldwide [1]. Accurate staging is desirable for treatment planning, since high-risk cancer is treated with surgery or radiation, while therapy for low-risk cancer considers active surveillance without invasive treatments. According to the new grading system proposed by the ISUP [2], low risk PCa are characterized by Grade Group (GG)=1,2 while high risk PCa are defined by GG$$$\geq$$$3. Diffusion Tensor Imaging (DTI) with high b-values (up to 2500s/mm2) has highlighted to provide a good discrimination between low- and high-risk PCa [3]. However, parameters derived from non-Gaussian diffusion model are in principle more sensitive to the microstructural changes in biological tissue than the Gaussian model [4]. Therefore, our aim was to compare the diagnostic performance of diffusional kurtosis imaging (DKI) and the conventional DTI approach in the discrimination between low- and high-risk PCa.Materials and Methods
A cohort of 24 patients with different aggressiveness grades (GG=1,2,3,4,5 corresponding to Gleason Score GS=3+3,3+4,4+3,4+4,4+5/5+4) PCa were retrospectively enrolled to be examined by MRI, using a 3T clinical MR scanner (Intera Achieva, Philips Medical Systems, The Nederlands) and a six-channel phased array SENSE torso coil. Each patient underwent the MR examination after two months from the first TRUS-guided biopsy. Diffusion-weighted images were acquired along 6 different diffusion directions with 6 different b-values (0,500,1000,1500,2000,2500 s/mm2), by using a diffusion weighted single shot EPI sequence (TR=3000, TE=67, FOV=150×130×70mm3, acquisition matrix=64×52, reconstruction matrix 96×96, slice thickness STK=3mm, gap=0, NSA=4). The acquisition protocol also included high spatial resolution T2-weighted (T2W) turbo spin echo (TR=3957, TE=150, turbo factor 21, FOV=150×130mm, STK=3mm, gap=0, acquisition matrix256x178, reconstruction matrix=512×512, NSA=6, flip angle=90°). The image pre-processing and the reconstruction of the Mean Diffusivity (MD) parametric maps was performed using FSL 5.0 (FMRIB Software Library v5.0, FMRIB, Oxford, UK). Parametric maps of apparent Kurtosis (K) and apparent diffusion coefficient (D) of the quadratic model were obtained by using an in-house algorithm developed in Matlab (MATLAB R2012b, The Mathworks, Natick, MA). Region of Interests (ROI) in PCa and contralateral benign zone were manually drawn by an expert radiologist, referring to T2W-images, for each subject. The pixels nearest to the PCa ROI edge were considered as peritumoral ROI. One-way ANOVA was performed to test statistical significance of differences in MD, K and D values calculated in PCa belonging to low- and high-grade groups. Moreover, the statistical significance of differences in MD, K and D values between benign and PCa tissue and between benign and peritumoral area were evaluated. The linear correlation between MD, K, D values and the tumour grade was estimated by the Pearson's test. Because low Signal to Noise Ratio (SNR) of DWIs acquired at larger b-values are an obvious drawback for non Gaussian diffusion techniques, we evaluated SNR of DWIs at each b values to investigate about the reliability DKI maps.Results
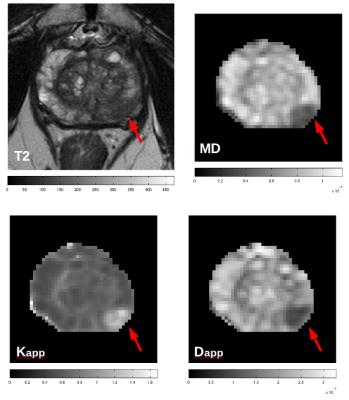
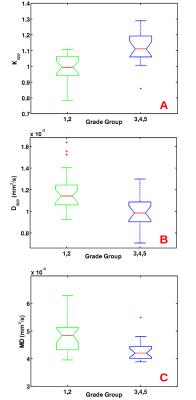
An example of T2, MD, K and D maps are displayed in Fig.1 for a patient with PCa characterized by GG=3. The SNR of b=0 images was approximately equal to 55 in PCa and remained higher than 22 up to b=2500 s/mm2. Statistically significant difference was found between each parameter values (MD, K, D) measured in PCa and benign controlateral zone. However, K had the highest significance (p<0.0001). K showed the highest significance (p<0.001) also in the discrimination between peritumoral and benign regions and peritumoral and PCa. A moderate positive correlation was found between K and GG (r=0.52; p<0.001), while a weak negative correlation was found between both D and MD and GG (r=-0.38, p=0.011; r=-0.36, p=0.016, respectively). Plots of K, D and MD as a function of GG are displayed in Fig.2. K, D and MD significantly discriminate between low-risk (GG=1&2) and high-risk PCa (GG=3,4,5) with p<0.001, p<0.004, p<0.02, respectively.Discussion and conclusions
The SNR was higher than 20, which is an acceptable value for considering DKI maps reliable. The diagnostic performance of DKI in discriminating between PCa and benign tissue and in differentiating among PCa characterized by different GG was superior compared to that provided by DTI. Moreover K maps better highlight differences between periferal PCa and benign tissue. In particular K discriminates between low- and high-risk PCa better than Mean diffusivity does (Fig.2).
These results confirm that non-Gaussian DKI parameters are more sensitive to tissue microstructural changes, occurring with tumour onset and progression compared to Gaussian parametrs. Therefore this work suggests that DKI could be a useful tool in the diagnosis and grading of PCa to ensure a correct therapy for the patients.
Acknowledgements
No acknowledgement found.References
[1] Ferlay, J. et al., Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer, 136 (2015): E359–E386.
[2] J.I. Epstein et al. The grading committee, the 2014 International Society of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am. J. Surg. Pathol., 40 (2) (2016), pp. 244–252.
[3] Nezzo, M. et al., Mean diffusivity discriminates between prostate cancer with grade group 1&2 and grade groups equal to or greater than 3. European Journal of Radiology , Volume 85 (2016) , Issue 10 , 1794 – 1801.
[4] Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed 2010;23:698– 710.
Figures