3177
Prognostic value of the pretreatment apparent diffusion coefficient (ADC) for outcome prediction of chemorefractory colorectal liver metastases undergoing 90-Yttrium Radioembolization1Department of Radiology, University Hospital Bonn, Bonn, Germany, 2Department of Nuclear Medicine, University Hopsital Saarland, Homburg/Saar, Germany
Synopsis
Imaging-based prediction of therapeutic response is highly desireable for further therapy decisions in patients with advanced malignancies. Therefore, we investigated whether pre-treatment values of the apparent diffusion coefficient (ADC) on diffusion-weighted MRI could predict the outcome of patients with liver-predominant metastatic colorectal cancer prior to 90-Yttrium microspheres radioembolization. Uni- and multivariate analyses were performed comparing various variables with potential impact on progression-free and overall survival. Our results reveal that pathologic pre-treatment ADC, alongside with established clinical parameters, is a strong and independent predicor of both progression-free and overall survival before RE treatment.
Purpose
The aim of this study was to investigate the role of the pretreatment mean apparent diffusion coefficient (ADC) on diffusion-weighted magnetic resonance imaging (DWI) as an imaging biomarker for the assessment of progression-free survival (PFS) and overall survival (OS) in patients with liver-predominant metastatic colorectal cancer (CRC) before application of radioembolization with 90-Yttrium microspheres (RE).Introduction
The use of RE is increasingly considered a therapeutic option for palliative treatment or tumor control of chemorefractory liver metastases, but is limited by its potential hepatotoxicity [1]. Knowledge whether patients are likely to benefit from RE is thus mandatory for clinical decision making. A reliable imaging-based prediction of therapeutic outcome and prognosis prior to therapy would be highly desirable and could affect treatment planning. Functional imaging techniques offer the ability to combine quantitative and qualitative assessment of tumor pathophysiology [2]. Together with other functional imaging techniques like positron emission tomography, the DWI derived ADC has been identified as a prognostic imaging biomarker for tumor characterization as well as for monitoring of early therapeutic response [3,4]. The question to what extent ADC could also provide prognostic information before treatment initiation with RE has, however, not been evaluated yet.
Methods
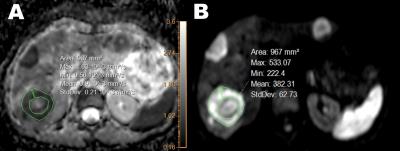
In this review-board approved study, 46 patients (17 women, mean age 61±11 years, range 45-82) with unresectable CRC liver metastases underwent RE and were retrospectively analyzed. All patients were included after failure of at least two lines of standard chemotherapy and had neither received other intra-arterial local therapies prior to RE nor adjuvant chemotherapy after the intervention. A routine clinical magnetic resonance examination which included DWI in axial orientation was performed 20±17 days before RE on the same 1.5T scanner system (Intera, Philips Healthcare, Best, The Netherlands): TR=1630 ms; TE=63 ms; b values 0,50,800 s/mm2; 256x256 acquisition matrix; 380 mm2 FOV; section thickness 7 mm; SENSE factor 2; fat-saturation by SPIR. Three target lesions per patient with diameters of at least 1 cm each were defined. Target lesions were manually contoured by placing circular regions-of-interest (ROI) within the viable component of the lesion while avoiding the inclusion of necrosis or fibrosis into the selected ROI (Figure 1). The results of ADC measurements within the three lesions were averaged for each patient. Progression-free (PFS) and overall survival (OS) were assessed from the first RE session and evidence of imaging progress or death of the patient was considered an event for PFS and OS, respectively. Survival stratification was performed with the log-rank-Test comparing various variables: Age ≥60 years, gender, hepatic tumor burden ≥25%, extrahepatic metastases, uni- vs. bilobar tumor extent and ECOG score ≥1. Additionally, Receiver Operating Characteristic (ROC) curves were plotted for the pre-treatment ADC values in order to discriminate cutoff values that yielded optimal sensitivity and specificity for PFS and OS. P<0.05 was considered statistically significant. Significant variables in univariate analysis were further evaluated using the multivariate Cox regression analysis to obtain hazard ratio estimates (HR) and 95% confidence intervals (CI).Results
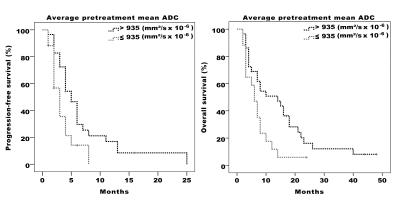
21 patients had extrahepatic disease, 27 had a hepatic tumor load ≥25% and 30 presented with bilobar involvement. A total of 138 target lesions were analyzed with a mean diameter of 5.42±2.26 cm. Median PFS and OS were 4 (CI 3-5) and 8 (CI 6-10) months after RE, respectively. ROC-analysis identified an ADC cut-off value of 935 mm2/s x 10-6 as the optimal threshold for distinguishing survivors from non-survivors. Patients with pre-treatment ADC values ≤935 mm2/s x 10-6 had both significantly shorter PFS (median 3 vs 5 months, p=0.022) and OS (median 6 vs 14 months, p=0.02) than patients with higher pre-treatment ADC (Figure 2). Among the remaining variables, hepatic tumor burden ≥25% was associated with both PFS (median 3 vs 6 months, p=0.015) and OS (median 6 vs 14 months, p=0.048) and ECOG score ≥1 was associated with OS (median 5 vs 8 months, p=0.045). In multivariate analysis, low pre-treatment ADC (PFS: HR=2.06, p=0.04) and high hepatic tumor burden (PFS: HR=2.11, p=0.037) were independently predictive of PFS, whereas low pre-treatment ADC remained the only independent predictor with significant impact on OS (OS: HR=2.09, p=0.036).Conclusion
ADC values as derived from DWI before the initiation of trans-arterial RE may have great potential as an imaging biomarker for prediction of both the therapeutic efficacy and survival in patients with liver-predominant metastatic CRC. ADC measurements can easily be adopted in clinical routine to facilitate the selection of patients who will most likely profit from RE and may thereby help optimizing the risk/benefit analysis of the individual patient.Acknowledgements
No acknowledgement found.References
1. Rühl R, Seidensticker M, Peters N, Mohnike K, Bornschein J, Schütte K, Amthauer H, Malfertheiner P, Pech M, Ricke J. Hepatocellular carcinoma and liver cirrhosis: assessment of the liver function after Yttrium-90 radioembolization with resin microspheres or after CT-guided high-dose-rate brachytherapy. Dig Dis 27:189–199.
2. Desar IM, van HerpenCM, van LaarhovenHW, Barentsz JO, Oyen WJ, van der Graaf WT. Beyond RECIST: molecular and functional imaging techniques for evaluation of response to targeted therapy. Cancer Treat Rev; 35:309–321.
3. Charles-Edwards EM, deSouza NM. Diffusion-weighted magnetic resonance imaging and its application to cancer. Cancer Imaging 2006; 6:135–143.
4. Ludwig JM, Camacho JC, Kokabi N, Xing M, Kim HS. The Role of Diffusion-Weighted Imaging (DWI) in Locoregional Therapy Outcome Prediction and Response Assessment for Hepatocellular Carcinoma (HCC): The New Era of Functional Imaging Biomarkers. Diagnostics 2015; 5:546-63.
Figures