3173
Elucidation of Male Urethral Sphincter Complex Using Diffusion Tensor Imaging (DTI) based Fiber-Tracking1VA Medical Center, San Diego, CA, United States, 2VA Medical Center, 3Physics, UC San Diego, San Diego, CA, United States, 4Radiology, UC San Diego, San Diego, CA, United States
Synopsis
Urethral sphincters play an important role in urinary incontinence, a major clinical problem affecting the aging population. We elucidate the anatomy of the urethral sphincter muscles pertinent to urinary continence function using in vivo, non-invasive proton-density and diffusion tensor imaging and DTI-based fiber tracking in young adults. Muscle fiber tracking consistently revealed, perhaps for the first time, the existence of two sphincter like muscles, with one proximal near the bladder neck and the other more distal, supporting the two sphincter concept to constrict/close the urethral opening with important implications for the effect of prostatectomy on urethral closure function.
Introduction
Urethral sphincters, including striated external (EUS) or rhabdo-sphincter, (as well as pelvic floor muscles), play an important role in urinary incontinence. This is a major clinical problem affecting a very large portion of the population, particularly as they age and their muscles degenerate. Prior to characterization of the age-related changes in the sphincters, the anatomical description of the normal male urethral sphincter has to be established. Such descriptions have undergone several revisions since it was first described more than 150 years ago, given previous conclusions were mostly derived from gross cadaveric dissection of adult male pelvis. These findings have been plagued by the inevitable accompanying distortions and alterations of the anatomical structures in cadavers. Our objective in this report was to elucidate the anatomy of the urethral sphincter muscles pertinent to urinary continence function by resorting to in vivo, non-invasive imaging, using proton-density, diffusion tensor imaging (DTI) and DTI-based fiber tracking in young adults and thereby improve our understanding by avoiding and minimizing the errors arising in cadaveric studies.Methods
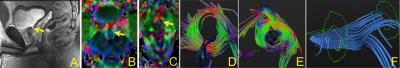
Five normal healthy male young subjects, (mean age ~25 yrs), after obtaining IRB consent, were scanned on a 3T GE MR scanner, using a multi-channel cardiac coil, lying supine, feet-first. After acquisition of a set of sagittal scout slices (Fig. 1.A), which clearly demarcated the urethra (arrow) in the mid-line slice, morphological axial high-resolution proton-density scans were acquired extending from a few slices below the base of the bladder to beyond the entry of the urethra into the penis (~18-22 slices depending on the height of the subject). DTI scans were obtained using a fat-suppressed single shot EPI sequence, with 32 non-collinear gradient directions with a b-factor of 400s/mm2, TE/TR of 57/~8000ms, 120x120 matrix, 23 FOV, 6 averages. 3 mm thick were acquired in the same position as the PD scans. These images were registered to baseline images to correct for eddy current and motion related artifacts, denoised using a Rician linear minimum mean square error. Tensor images were calculated using a Gaussian model of diffusion, followed by correction for susceptibility based distortion artifacts and finally processed to generate the eigenvalue and fractional anisotropy and tensor images (Fig. 1.B). Fiber tracking was performed using either DTIStudio1 or DTITools2, with ‘fiber assignment by continuous tracking’ (FACT) algorithm, and stopping criteria of FA< 0.12~0.15. ROI’s were placed in the tensor slices where the annular rings of the sphincter muscles were visualized, and fibers tracked to construct fibers within this urethral complex.Results
Excellent DTI images could be obtained using the above protocol, with little distortion as revealed by a visual check of the error color maps between the reoriented images and the original ones. However, in some subjects, there was some amount of distortion, though not prohibitive, caused by the susceptibility mismatch between the air in the rectal canal and adjacent tissue. FA values, and the different eigenvalues were determined. A consistent finding over all the subjects was that of possibly two sphincter like muscles (Fig. 1.D-E), with one proximal near the bladder neck (arrow in Fig. 1.B) and the other more distal (Fig.1.C). In the tensor images, a conventional coloring scheme was utilized, with blue color indicating fibers oriented superior-inferior, red left-right while green anterior-posterior directions. Fiber tracking of these tensor images yielded fiber structures within the urethral sphincter complex. The proximal sphincter is shown in Fig. 1.D, the distal sphincter in Fig. 1.E. The connecting superior-inferior longitudinal fibers are shown in blue in Fig. 1.F. A consistent finding was the distance between the two sphincters is between 21 and 24 mm (i.e., 7 to 8 slices) in all subjects.Discussion and Conclusion
This is perhaps the first DTI and fiber tracking of the male urethral sphincter complex. Our findings support the two sphincter concept to constrict/close the urethral opening. Our results have important implications for the effect of prostatectomy on urethral closure function and the currently used surgical techniques for prostate surgery. These observations will form the basis of normal urethral morphology that can be monitored in post-surgery patients and correlated with age /urinary incontinence symptoms.Acknowledgements
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant 5RO1-AR-053343-08References
1. https://www.dtistudio.org/
2. www.bmia.bmt.tue.nl/Software/DTITool
Figures