3152
Free-Breathing Self-Navigated Isotropic 3-D CINE Imaging of the Whole Heart using Adaptive Triggering and Retrospective Gating1Pattern Recognition Lab, Department of Computer Science, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 2Erlangen Graduate School in Advanced Optical Technologies (SAOT), Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 3Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
We present a method for free-breathing whole-heart 3-D CINE imaging based on adaptive triggering and retrospective gating and compare it to a previously published method using prospective triggering. We show that our method is simultaneously robust to heart-rate variability during the scan and able to cover the entire cardiac cycle, which is not the case for prospective triggering. A validation in 6 volunteers shows reduced end-diastolic volume bias compared to a gold-standard 2-D reference for our method. Image reconstruction is integrated into the scanner system and takes less than 3 minutes.
Purpose
In clinical practice, cardiac function parameter estimation is based on multi-breath-hold 2-D CINE imaging, featuring high in-plane resolution, but thick slices. 3-D CINE acquisitions with isotropic resolution have been proposed,1-3 but require long reconstruction times,1 long breath-holds2 or prospective triggering (PT) to synchronize the acquisition to the heartbeat.2,3 However, PT may not be able to acquire the entire cardiac cycle and can cause image artifacts in the presence of heart-rate variability. In this work, we extend a recent method3 to use adaptive triggering and retrospective gating (ATRG), which may solve these issues.Methods
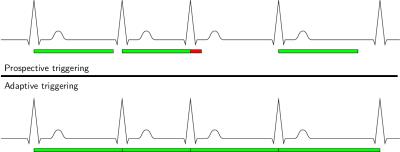
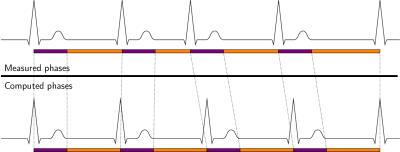
In PT acquisitions, a fixed acquisition window for each heartbeat is defined before the scan is started. While PT with a short acquisition window is commonly used in case of cardiac arrhythmia, the late diastolic phase of the cardiac cycle might be missed and PT provides no guarantee that actual cardiac state and assigned phase for reconstruction correspond (see Figure 1, top). ATRG allows for a continuous acquisition in the presence of heart-rate variability, where the acquisition window for each heartbeat matches its length, but leads to a varying number of cardiac phases for each heartbeat (see Figure 1, bottom). Retrospective gating of this data to a reference heartbeat is commonly done by linear interpolation, which can cause artifacts in the presence of arrhythmia. To prevent artifacts due to arrhythmia and ensure correlation between actual cardiac state and assigned phase, we selected a model where the first $$$300\,\text{ms}$$$ correspond to a fixed-length systole and the remaining time is a variable-length diastole which is linearly stretched to the reference heartbeat (see Figure 2).
The previously proposed sampling pattern3 is well suited for ATRG because of the golden-angle increment of the spiral arms, allowing retrospective rebinning while ensuring sufficient k-space coverage.
Whole-heart, free-breathing 3-D CINE imaging with PT and ATRG in short-axis (SA) orientation was performed in $$$6$$$ healthy volunteers ($$$2$$$ female, age $$$27\pm{}4$$$) on a $$$1.5\,\text{T}$$$ clinical MR scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). A 3-D volume-selective bSSFP prototype imaging sequence with the following parameters was used: TR $$$2.6\,\text{ms}$$$, TE $$$1.3\,\text{ms}$$$, $$$\alpha = 39^\circ$$$, FOV $$$365\times{}(320\pm{}28){}\times{}152\,\text{mm}^3$$$, acquired voxel size $$$(1.9\,\text{mm})^3$$$, measured temporal resolution $$$42\,\text{ms}$$$, $$$20$$$ computed phases, fixed acceleration factor of $$$3.1$$$ compared to the fully-sampled matrix and a receiver bandwidth of $$$1000\,\text{Hz/px}$$$. For signal reception, $$$18+12$$$ elements of an anterior $$$+$$$ posterior local coil matrix were used. For reference, a $$$12$$$-slice SA 2-D bSSFP acquisition with $$$\alpha = 54^\circ$$$ and retrospective ECG gating in multiple breath-holds was performed to cover the LV with similar temporal resolution, identical in-plane resolution and a slice thickness of $$$8\,\text{mm}$$$.
Compressed sensing reconstruction was performed as previously presented,3,4 with one modification in the regularization term. While redundant wavelets were used previously, orthogonal wavelets and cycle spinning were employed in our method to retain translation invariance while reducing the memory footprint and required computation time. The image reconstruction was fully integrated into the scanner software and multi-GPU accelerated.
For evaluation, we compared the acquisition and reconstruction times, ventricular function (VF) parameters computed manually from the images of the gold standard 2-D CINE and PT and ATRG 3-D CINE in corresponding slices of all data sets as well as the Hausdorff distance5 of the segmented blood pool in the first and last phases of PT and ATRG acquisitions to measure if the entire cardiac cycle was captured.
Results and Discussion
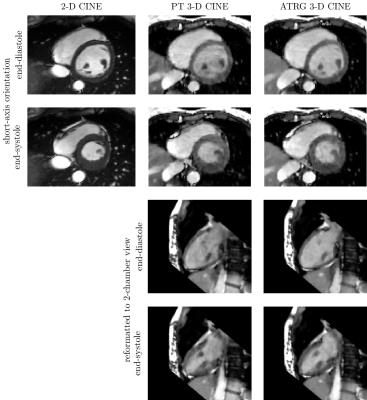
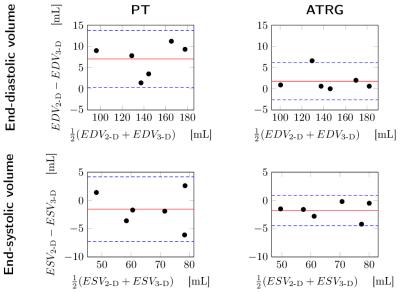
Five out of six volunteers showed considerable heart rate variability leading to skipped triggers even for conservatively selected acquisition windows. Acquisition time was $$$4\,\text{min}\,43\,\text{s}\pm{}32\,\text{s}$$$, reconstruction time was $$$2\,\text{min}\,38\,\text{s}\pm{}27\,\text{s}$$$ for ATRG and comparable for PT. For qualitative results, see Figure 3. Bland-Altman plots for VF parameters are shown in Figure 4. The Hausdorff distance between first and last phase was $$$5.5\pm{}1.1\,\text{mm}$$$ for PT and $$$3.4\pm{}0.9\,\text{mm}$$$ for ATRG.
ATRG acquisitions show less EDV bias because the entire diastole is acquired, also confirmed by the smaller Hausdorff distance. The EDV bias of PT is in line with other published results.6 With ATRG, it is not necessary to choose an appropriate acquisition window. The use of the orthogonal wavelet transform and cycle spinning leads to a reduction of $$$57\,\%$$$ in reconstruction time compared to previously published work.3
Conclusion
With ATRG, 3-D CINE imaging can be performed without the need to select an appropriate acquisition window and is robust to heart-rate variation. Coverage of the entire cardiac cycle can be achieved, leading to reduced VF bias. Full scanner integration and reconstruction times of less than $$$3\,\text{min}$$$ improve the clinical relevance of the presented method.Acknowledgements
The authors gratefully acknowledge funding of the Erlangen Graduate School in Advanced Optical Technologies (SAOT) by the German Research Foundation (DFG) in the framework of the German excellence initiative.References
1S. Coppo et al. "Free-running 4D whole-heart self-navigated golden angle MRI: Initial results". Magn. Reson. Med. 74(5):1306-1316, 2015.
2J. Wetzl et al. "Isotropic 3-D CINE Imaging with Sub-2mm Resolution in a Single Breath-Hold". Proc. ISMRM #1011, 2015.
3J. Wetzl et al. "Free-Breathing, Self-Navigated Isotropic 3-D CINE Imaging of the Whole Heart Using Cartesian Sampling". Proc. ISMRM #411, 2016.
4J. Liu et al. "Dynamic cardiac MRI reconstruction with weighted redundant Haar wavelets". Proc. ISMRM #178, 2012.
5R.T. Rockafellar et al. "Variational Analysis". Springer Berlin Heidelberg, 2009.
6G. Vincenti et al. "Compressed Sensing Single-Breath-Hold CMR for Fast Quantification of LV Function, Volumes, and Mass". JACC, 7(9):882-92, 2014.
Figures