3132
Simultaneous Bright and Black Blood Whole Heart Phase Sensitive Inversion Recovery (PSIR) for Non Contrast Enhanced Coronary Lumen and Plaque Characterization1Division of Imaging Sciences and Biomedical Engineering, King’s College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Synopsis
This study introduces a 3D whole-heart sequence with image-based navigation for non-contrast enhanced coronary lumen and coronary thrombus/haemorrhage characterization. The sequence is based on a phase sensitive inversion recovery (PSIR)-like approach enabling the acquisition of two bright-blood datasets for visualization of the coronary lumen and estimation of motion parameters. Furthermore, a third, fully co-registered dark-blood dataset for thrombus/haemorrhage characterization is obtained from the PSIR reconstruction. With the proposed approach the coronary lumen is visualized with improved SNR/CNR and vessel sharpness when compared to a conventional T2 prepared coronary MRA acquisition. In addition, effective blood signal suppression and feasibility for thrombus visualization is shown with the PSIR reconstructed black-blood dataset.
Introduction
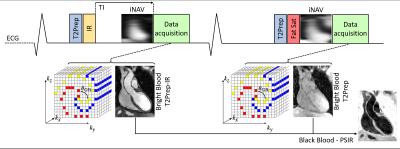
MRI has shown great potential for coronary lumen and thrombus/haemorrhage visualization using bright-blood coronary MRA (CMRA) and T1-weighted black-blood sequences1,2. However, these sequences traditionally suffer from limited volumetric coverage and unpredictable scan times due to the use of simplified respiratory motion gating. Moreover, the fusion of coronary lumen and thrombus/haemorrhage images can be affected by motion between these sequential acquisitions. A recently introduced whole-heart CATCH sequence3 addresses some of these drawbacks by acquiring an Inversion-Recovery (IR)-prepared black-blood volume and a bright-blood volume usable as anatomical reference in an alternate fashion. With this approach, however, respiratory motion parameters cannot be directly extracted from the black-blood image and, in addition, the anatomical reference might not provide adequate diagnostic information. Here, we propose a 3D, non-contrast enhanced, bright-blood and black-blood phase sensitive IR (PSIR)4 sequence with image-based navigation5 for simultaneous coronary lumen and thrombus/haemorrhage visualization. This is achieved by alternating a bright-blood T2-prepared IR (T2prep-IR, odd heartbeats) and a bright-blood T2-prepared (T2prep, even heartbeats) whole-heart acquisition that are then combined in a PSIR-like reconstruction4 to obtain a black-blood volume (Fig1). The proposed approach 1) enables the acquisition of two different bright-blood datasets providing anatomical information (coronary lumen) and from which respiratory motion information can be extracted independently, 2) provides a co-registered black-blood PSIR dataset for thrombus/haemorrhage visualization, 3) introduces intrinsic robustness with respect to the choice of the inversion time (TI) by exploiting the PSIR framework, and 4) ensures 100% scan efficiency and predictable scan time by correcting for respiratory motion with image-based navigation5. The proposed sequence was validated in a standardized phantom and compared to conventional CMRA and PSIR4,6 sequences in healthy subjects.Methods
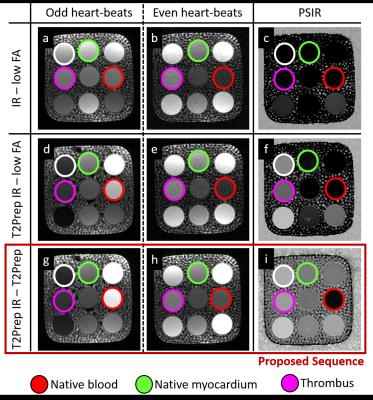
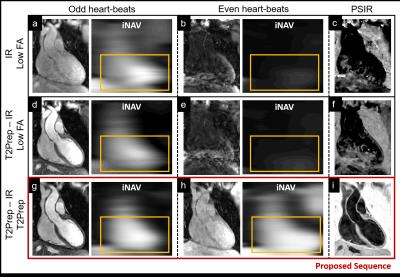
Sequence Design: A whole-heart, ECG-triggered, bSSFP, Cartesian prototype sequence with spiral profile order7 was implemented and adapted to perform PSIR-like acquisitions as in Fig1. A 2D image-navigator (iNAV)5 is acquired at each heartbeat for respiratory motion estimation/correction. Data acquisition was performed on a 1.5T system (Siemens MAGNETOM Aera). Imaging parameters include: resolution=1x1x2mm, FOV=320x320x80-130mm, coronal orientation, TR/TE=3.6ms/1.56ms, flip-angle=90deg, TI=110ms, T2Prep duration=40ms. Even heartbeat acquisitions included a SPIR pulse for fat saturation, while a STIR-like fat suppression was employed in odd heartbeats. Phantom: A standardized T1/T2 phantom8 mimicking myocardium, blood and thrombus was used. Data acquisition was performed with 1) a conventional PSIR sequence interleaving the acquisition of an IR dataset with a low flip-angle reference image, as in Reference 4 (4), 2) a PSIR sequence interleaving a T2Prep-IR module with a low-flip angle reference image, as in Reference 6 (6) and 3) the proposed sequence, as in Fig1. Healthy subjects: Data were acquired in one healthy volunteer using the same PSIR sequences as for the phantom acquisition. Furthermore, data were acquired in 7 additional healthy subjects using the proposed sequence (~18min) and a conventional CMRA scan with matching imaging parameters for comparison purposes (~9min). Motion estimation and image reconstruction: iNAVs belonging to both bright-blood datasets were used in order to estimate translational motion along the superior-inferior and left-right direction. The two datasets were separately corrected, reconstructed and then co-registered prior to PSIR reconstruction. Data analysis: T2prep-IR dataset (odd heartbeats) enables high-contrast coronary lumen visualization with the proposed approach and was compared with conventional CMRA acquisition in terms of vessel length (VL) and percentage vessel sharpness (%VS) for both right and left coronary arteries (RCA and LAD), and in terms of SNR of blood and CNR blood/myocardium9. %VS and VL were also quantified on the T2Prep-IR datasets before and after motion correction.Results
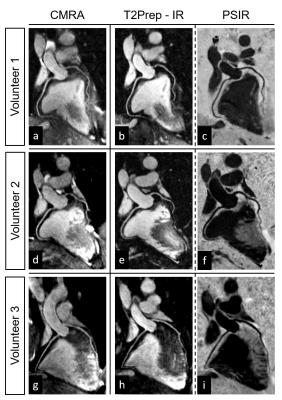
Acquisitions in phantom and healthy subjects showed the effectiveness of the proposed sequence to provide improved contrast and motion estimation when compared to previously proposed PSIR frameworks (Fig2, Fig3). For the T2Prep-IR datasets (designed for coronary lumen visualization), VL and %VS significantly improved after motion correction (%VS-LAD: 33.8±21.6% vs 50.1±7.5%, %VS-RCA: 44.4±7.7 vs 50.7±6.1, VL-LAD: 6.1±5.1cm vs 9.6±4.4cm, VL-RCA: 7.1±0.9cm vs 8.7±2.0cm, P<0.05) (Fig4). When compared to the conventional CMRA acquisition, the T2Prep-IR dataset showed higher SNR and CNR (19.2±1.3 vs 22.8±3.1, and 9.2±0.3 vs 15.5±2.8; P<0.05), thus leading to significantly improved coronary delineation (for CMRA: %VS-LAD: 33.2±3.6%, %VS-RCA: 43.7±6.8% -P<0.05- VL-LAD: 9.6±1.5cm, VL-RCA: 8.1±1.9cm) (Fig5).Conclusion
A new sequence for non-contrast enhanced PSIR-like reconstruction was developed, which provides two bright-blood datasets for coronary lumen visualization and a co-registered black-blood dataset for thrombus/haemorrhage detection. Coronary delineation provided in the T2Prep-IR dataset was superior to that of conventional CMRA. Future studies will include the use of an accelerated non-rigid respiratory motion correction framework10, and clinical validation in patients with coronary artery disease.Acknowledgements
This work was supported by EPSRC EP/N009258/1, EP/P001009/1 and MRC MR/L009676/1.References
1) Jansen CH, et al. Detection of intracoronary thrombus by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 2011; 124: 416-24.
2) Noguchi T, et al. High-intensity signals in coronary plaques on non-contrast T1-weighted magnetic resonance imaging as a novel determination of coronary events. J Am Coll Cardiol 2014; 63: 989-99.
3) Yibin X, et al. Coronary atherosclerosis T1-weighed characterization with integrated anatomical reference: comparison with high-risk plaque features detected by invasive coronary imaging. JACC Cardiovascular Imaging; In press – corrected proofs.
4) Kellman P, et al. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med 2002; 47(2): 372-83.
5) Henningsson M, et al. Whole-heart coronary MR angiography with 2D self-navigated image reconstruction. Magn Reson Med 2012; 67: 437-45.
6) Xie J, et al. 3D Flow-Independent Peripheral Vessel Wall Imaging Using T2-Prepared Phase-Sensitive Inversion-Recovery SSFP. J. Magn. Reson. Imaging 2010; 31: 248-54.
7) Prieto C, et al. Highly efficient respiratory motion compensated free-breathing coronary MRA using golden-step Cartesian acquisition. J. Magn. Reson. Imaging 2015; 41: 738-46.
8) Captur G, et al. A T1 and ECV phantom for global T1 mapping quality assurance: The T1 mapping and ECV standardisation in CMR (T1MES) program. Journal of Cardiovascular Magnetic Resonance 2016; 18(Suppl 1): W14.
9) Etienne A, et al. “Soap-Bubble”: Visualization and quantitative analysis of 3D coronary magnetic resonance angiograms. Magn Reson Med 2002; 48:658-66.
10) Cruz G, et al. Highly efficient non rigid motion-corrected 3D whole heart coronary vessel wall imaging. Magn Reson Med 2016; DOI: 10.1002/mrm.26274.
Figures