3127
3D segmentation of the Pulmonary Arteries on Magnetic Resonance Angiography1Academic Radiology, The University of Sheffield, Sheffield, United Kingdom, 2Insigneo, Institute of In-Silico Medicine, Sheffield, United Kingdom
Synopsis
Contrast enhanced magnetic resonance angiography (MRA) is in common use in the assessment of pulmonary hypertension. We present a novel method for segmentation of the pulmonary arteries allowing rapid assessment of the proximal pulmonary arteries down to the 4th generation. Whilst the strongest correlation with mean pulmonary artery pressure was with the right main pulmonary artery, the torsion of the 4th generation pulmonary arteries was also associated with increasing mean pulmonary artery pressure. We hope quantitative vessel segmentation will improve our understanding of the impact of proximal pulmonary arterial remodelling in pulmonary hypertension.
PURPOSE:
Contrast enhanced magnetic resonance angiography (MRA) is commonly used to assess the pulmonary vasculature in patients with suspected chronic thrombo-embolic disease (CTEPH), particularly to assess proximal surgically treatable disease (1). There is growing interest in the quantitative assessment of the pulmonary vascularity in pulmonary vascular disease, but with little work in 3D MR pulmonary angiography. MRA has the advantage over CT due to good arterial and venous separation. The aim of this work was to develop a novel methodology for the segmentation of pulmonary arteries on MRA.METHODS:
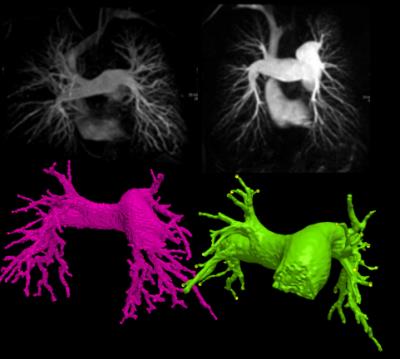
Consecutive patients who underwent pulmonary MRA as part of the routine clinical pathway at a tertiary pulmonary hypertension referral centre were reviewed (2). The imaging was split into 2 parts: initially an unenhanced T1 volume was acquired. A bolus-tracking scan was used to assess the optimal delay to pulmonary arterial enhancement. A bolus of 0.1mmol/kg Gadovist was then administered at 5ml/sec. A 3D dataset was acquired of the chest and the MRA was generated as a subtraction of the non-enhanced dataset from the contrast enhanced dataset. Pulmonary artery segmentations were created using ScanIp 2016.09 (Synopsis, San Francisco) (figure 4). The process is semi-automated, involving thresholding and region growing. The vessel edges were defined as the signal intensity at “full-width-half-maximum” of the right pulmonary artery. Once segmentation was completed centreline statistics were generated to derive length, cross sectional area and curvature of the pulmonary arterial tree. Data was analysed using Matlab R2016A (Mathworks, Natick MA) and SPSS (IBM, Armonk NY). The median of each centreline cross sectional areas was used to ensure exclusion of outliers. Beyond the left and right pulmonary artery the data was presented as a median per generation. The accuracy of the segmentation was assessed with scatter and Bland-Altman plots of the diameter of the main, left and right pulmonary arteries from the segmentation against the traditional clinical measurement made on axial imaging.RESULTS:
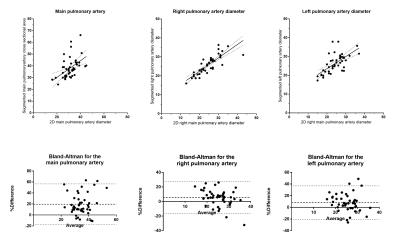
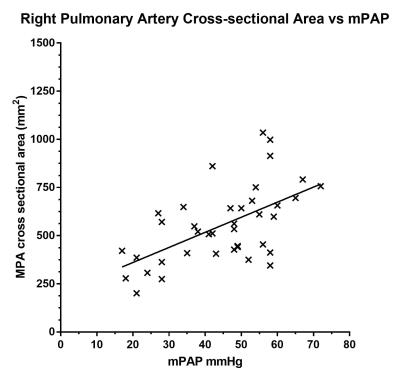
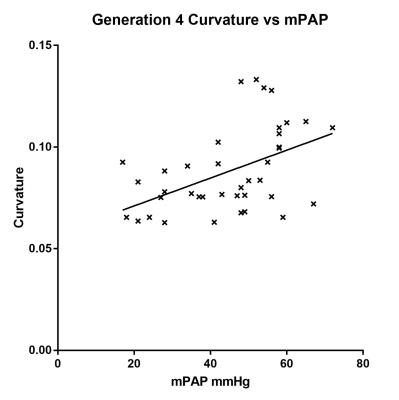
48 cases were segmented: 3 patients had no pulmonary hypertension (PH) and the other 45 had different severities of PH. The best agreement between manual and 3D measurement of arterial diameter was achieved with the right pulmonary artery (r2=0.25, 0.70 and 0.42 for the main, right and left pulmonary arteries respectively). Bland-Altman plots show the 3D segmentations overestimate the diameter, with a bias of 10-20% (figure 2). There was a significant correlation between the right main pulmonary artery cross sectional area and mean pulmonary artery pressure (mPAP) (r2=0.35, p<0.0001) (figure 3). A scatter plot of cross-sectional area against mPAP showed reducing correlations for subsequent generations (gen3 r=0.262, p=0.10, gen4 r = 0.24, p=0.14). A correlation between the mean curvature of the 4th generation vessels and mPAP (r=0.49 p=0.001) was also found (figure 4). This correlation was not present in the more proximal generations.DISCUSSION:
We propose a novel semi-automated method for the 3D segmentation of pulmonary arteries on MRA, which takes approximately 10 minutes. The segmentation was generated down to at least the 4th generation. The size of vessels appears to be overestimated with respect to the traditional measurement, likely explained by partial voluming effect from the relatively low resolution of MRA (typical voxel size 0.9x0.9x1.4mm3). Motion during the cardiac cycle is another potential error, since the MRA was not ECG gated. The anatomy of the left and right pulmonary arteries likely explains why the right pulmonary artery yields a more reproducible measurement. The right pulmonary artery has a straight course, whereas the left curves over the left main bronchus. Due to the curvature, the left pulmonary artery diameter is difficult to measure, and may be compressed by external structures. Unfortunately, the main pulmonary artery was often cut off the edge of the field of view so was not easily measured. Of particular interest is the correlation of the curvature of the 4th generation arteries with mPAP. This has potential to explain the underlying pathophysiology in patients with pulmonary hypertension, and may reveal a stronger relationship when the patients are further phenotyped. We will also work to further improve the segmentation algorithm and compare with CT segmentation. The use of BSSFP 3D MRA will also be useful for the assessment of wall adherent clot (3).CONCLUSION:
Segmentation of the pulmonary arteries on MRA is fast and easy to perform. It is more accurate for the right main pulmonary artery, likely due to its anatomy. The curvature of the 4th generation vessels increases with increasing mean pulmonary arterial pressure. With improvements to the segmentation process, this method shows potential for further research into the pathophysiology and phenotyping of pulmonary hypertension.Acknowledgements
This work presents independent research funded by the National Institute for Health Research (NIHR) and the MRC. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
1. Rajaram S, Swift AJ, Telfer A, Hurdman J, Marshall H, Lorenz E, et al. 3D contrast-enhanced lung perfusion MRI is an effective screening tool for chronic thromboembolic pulmonary hypertension: results from the ASPIRE Registry. Thorax [Internet]. 2013;68(7):677–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23349220
2. Hurdman J, Condliffe R, Elliot CA, Davies C, Hill C, Wild JM, et al. ASPIRE registry: Assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J [Internet]. 2012 Apr;39(4):945–55. Available from: http://erj.ersjournals.com/lookup/doi/10.1183/09031936.00078411
3. Rajaram S, Swift AJ, Capener D, Telfer A, Davies C, Hill C, et al. Diagnostic accuracy of contrast-enhanced MR angiography and unenhanced proton MR imaging compared with CT pulmonary angiography in chronic thromboembolic pulmonary hypertension. Eur Radiol [Internet]. 2012;22(2):310–7. Available from: http://link.springer.com/10.1007/s00330-011-2252-x
Figures