3122
Quantitative Multi-Contrast Atherosclerosis Characterization (qMATCH): Comprehensive Quantitative Evaluation of Atherosclerosis in a Single-Scan1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Department of Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States
Synopsis
Although MRI is an attractive imaging modality for the evaluation of carotid atherosclerosis thanks to its versatility and noninvasiveness, its current clinical usage is still limited. Major drawbacks of conventional protocols include long scan time and observer variability due to the qualitative nature of the images. In this
Purpose
MRI is a preferred imaging modality for the evaluation of carotid atherosclerosis, with the capability to provide multi-faceted diagnostic information on both luminal stenosis as well as plaque composition through various image contrasts1. Recently, we developed the MATCH technique2 as a single-scan solution for multi-contrast carotid imaging, offering much shortened exams and co-registered images. Because of their qualitative nature, however, multi-contrast images from MATCH still suffer from the same intra/inter-observer variability as conventional protocols. Quantitative mapping of the carotid vessel wall potentially offers high reproducibility and portability of the results3,4. In this study, we extended the concept of MATCH to develop an accelerated MR technique for comprehensive evaluation of carotid atherosclerosis (including bright-blood MRA, dark-blood wall images, multiple T1/T2 weightings and quantitative mapping) in a single scan under 8 minutes.
Methods
We designed the qMATCH technique based on low-rank tensor (LRT) framework5,6 which exploits the partial separability of space and contrast dimensions in the multi-contrast images to achieve vast acceleration.
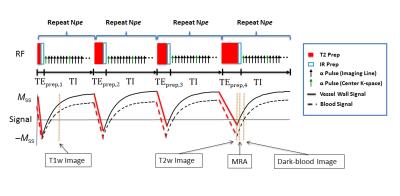
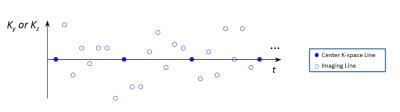
Sequence implementation: qMATCH employed 3D flow-compensated spoiled gradient echo readout with variable-duration T2-IR preparations to generate T1 and T2 contrast (Fig.1). Cartesian acquisition with randomized reordering in ky and kz directions was implemented according to a variable-density Gaussian distribution (Fig.2). The center k-space line was collected every eighth readout to serve as LRT training data6.
Image reconstruction: We represent our 5-D image $$$I(x,y,z,T_{I},T_{E})$$$ as a 3-way tensor with dimensions indexing voxel location $$$\mathbf{r}=(x,y,z)$$$, inversion time $$$T_{I}$$$, and T2prep-duration $$$T_{E}$$$. Due to the strong correlation between images with different contrasts, this tensor is low-rank and can therefore be expressed in collapsed form as $$$\mathbf{U\Phi}$$$, where $$$\mathbf{\Phi}$$$ contains basis functions describing T1/T2 relaxation and $$$\mathbf{U}$$$ contains spatial coefficients. We perform image reconstruction by determining $$$\mathbf{\Phi}$$$ from training data and then fitting it to the remainder of the sparsely sampled data:$$\hat{\mathbf{U}} = \arg\min_{\mathbf{U}}\|\mathbf{d}-\mathrm{E}(\mathbf{U\Phi})\|_2^2+\lambda\mathrm{TV}(\mathbf{U}), $$ where $$$\mathbf{d}$$$ is the measured data, $$$\mathrm{E}(\cdot)$$$ describes MRI encoding and sampling, $$$\mathrm{TV}(\cdot)$$$ is the total variation regularization functional, and is the regularization parameter.
Imaging protocol: All data were acquired on a 3T Siemens Verio scanner with the following parameters: coronal orientation, spatial resolution = 0.7mm isotropic, FOV=150x150x26mm3,$$$\alpha = 8^\circ$$$ ,TR = 2.08s, TEs = 20/30/40/50/60/70ms, scan time = 7.5mins. qMATCH was tested in relaxometry phantoms7 made of nickel chloride (for T1) and agarose (for T2). In vivo imaging was performed in 7 normal subjects without known carotid atherosclerosis. Standard IR spin echo was used as the reference in the phantom studies. MOLLI8 and T2prep SSFP9 was used as the reference in vivo.
Results
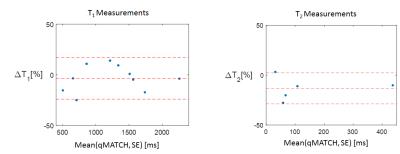
Figure 3 shows the Bland-Altman plot comparing the T1 and T2 quantification results between qMATCH and spin echo reference in the phantom studies.
All human subjects successfully completed the study. Figure 4 shows a representative qMATCH image set from a normal subject without known carotid atherosclerosis. MRA, dark-blood vessel wall, T1-weighted, and T2-weighted images are demonstrated as the corresponding phases noted in Figure 1. T1 and T2 maps were quantified.
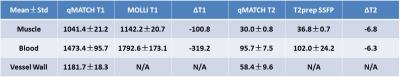
Table 1 summarizes the T1 and T2 measurements for muscle, blood and vessel wall by qMATCH and 2D reference techniques, respectively, and the relative differences between the two methods.
Discussion
Preliminary results from phantoms and normal subjects demonstrated excellent multi-contrast image quality and reliable T1 and T2 quantification by qMATCH. High-resolution 3D coronal acquisition allowed large coverage and flexible viewing. All qMATCH images in a set are inherently co-registered which may simplify their usage in a clinical setting. Some luminal blood signal inhomogeneity and T1 errors were likely due to inflow effects.Conclusion
The proposed qMATCH technique is a promising method for comprehensive evaluation of carotid atherosclerosis in a single scan. It has the potential to provide integrated assessment of multiple lesion characteristics including luminal stenosis (by bright-blood MRA), plaque burden (by dark-blood wall images), and plaque composition (by multiple T1/T2 weightings and quantitative mapping). Future work will focus on application and validation in patients with carotid atherosclerosis.Acknowledgements
No acknowledgement found.References
1 Cai, J. M. et al. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 106, 1368-1373 (2002).
2 Fan, Z. et al. Multi-contrast atherosclerosis characterization (MATCH) of carotid plaque with a single 5-min scan: technical development and clinical feasibility. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 16, 53, doi:10.1186/s12968-014-0053-5 (2014).
3 Biasiolli, L., Lindsay, A. C., Chai, J. T., Choudhury, R. P. & Robson, M. D. In-vivo quantitative T2 mapping of carotid arteries in atherosclerotic patients: segmentation and T2 measurement of plaque components. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 15, 69, doi:10.1186/1532-429X-15-69 (2013).
4 Coolen, B. F. et al. Three-dimensional quantitative T1 and T2 mapping of the carotid artery: Sequence design and in vivo feasibility. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 75, 1008-1017, doi:10.1002/mrm.25634 (2016).
5 He, J. et al. Accelerated High-Dimensional MR Imaging With Sparse Sampling Using Low-Rank Tensors. IEEE transactions on medical imaging 35, 2119-2129, doi:10.1109/TMI.2016.2550204 (2016).
6 Christodoulou, A. G. et al. Fast dynamic electron paramagnetic resonance (EPR) oxygen imaging using low-rank tensors. Journal of magnetic resonance 270, 176-182, doi:10.1016/j.jmr.2016.07.006 (2016).
7 Stanisz, G. J. et al. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 54, 507-512, doi:10.1002/mrm.20605 (2005).
8 Messroghli, D. R. et al. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 52, 141-146, doi:10.1002/mrm.20110 (2004).
9 Giri, S. et al. T2 quantification for improved detection of myocardial edema. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 11, 56, doi:10.1186/1532-429X-11-56 (2009).
Figures