3120
Whole-brain vessel wall imaging within 5 minutes using compressed sensing accelerated IR-SPACE1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Siemens Healthcare, Los Angeles, CA, United States, 3Imaging, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 4Xuanwu Hospital, Beijing, People's Republic of China, 5Neurology, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 6Neurosurgery, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 7Siemens Healthcare, Erlangen, Germany
Synopsis
Inversion-recovery (IR) prepared SPACE was recently proposed as a whole-brain intracranial vessel wall imaging technique. This work aimed to investigate the feasibility of accelerating the scan from 8 min to <5 min using compressed sensing (CS). A prototype CS IR-SPACE sequence was implemented on a 3T system. Wavelet sparse regularization (λ) and iteration (Iter) were optimized for the scenario of sampling 15% of k-space data based on a volunteer study. Image quality was visually comparable between CS IR-SPACE and regular IR-SPACE scans. In addition, CS IR-SPACE and IR-SPACE showed comparable lesion delineation quality in patients despite markedly different scan times.
INTRODUCTION
High-resolution black-blood MRI using variable-flip-angle 3D fast spin-echo has emerged as a promising intracranial vessel wall (IVW) imaging technique [1,2]. Recently, inversion-recovery (IR) prepared SPACE (IR-SPACE) was proposed as an improvement of this approach, providing remarkable suppression of cerebrospinal fluid (CSF) signals, enhanced T1 contrast weighting, and most importantly, whole-brain spatial coverage [3]. A parameter tune-up strategy currently allows a IR-SPACE scan to be completed within 7-8 min [4]. Further acceleration in data acquisition would be beneficial for wider clinical adoption of the technique. This work aimed to investigate the feasibility of whole-brain IVW imaging within 5 min using compressed sensing accelerated IR-SPACE.METHODS
A prototype IR-SPACE sequence with compressed sensing acceleration (CS IR-SPACE) and online reconstruction was implemented on a 3T system (MAGNETOM Skyra, Siemens Healthcare) equipped with a 20-channel head-neck coil. Data acquisition followed a variable-density Poisson-disk random undersampling pattern in the Ky-Kz plain. Image reconstruction was performed by enforcing sparsity in the wavelet space and using a fast iterative soft-thresholding algorithm with a redundant 3D Haar wavelet transform [5]. Two factors, i.e. wavelet sparse regularization (λ) and iteration (Iter), were specifically used in this work to optimize overall image quality.
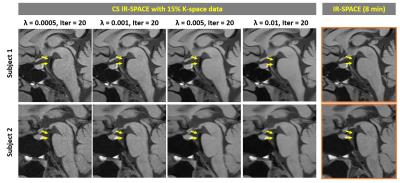
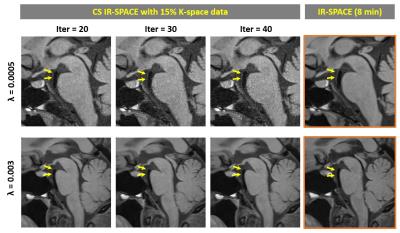
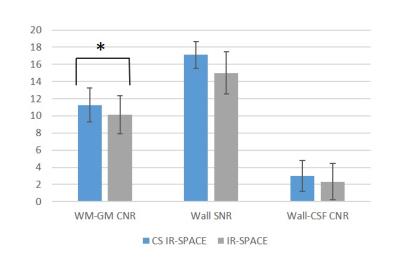
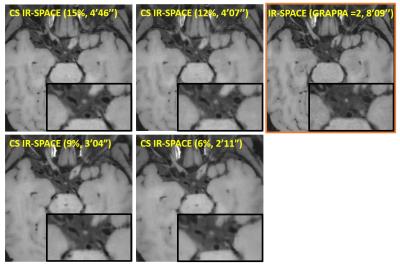
For the IRB-approved study, 7 healthy volunteers (4F, age 24-38 yrs) and 5 patients (3F, age 46-71 yrs) scheduled for routine MRA examinations due to suspicious cerebrovascular disease were recruited with written informed consent. To meet the demand on spatial resolution and slice number (elaborated below), a whole-brain scan within 5 min mandates 15% or less of k-space data to be acquired with the current prototype sequence. Therefore, accelerated imaging was first attempted with 15% of k-space; in 2 healthy subjects, the effects of λ and Iter on overall image quality were investigated by repeating image reconstruction retrospectively with a series of values (λ = 0.0005, 0.0008, 0.001, 0.003, 0.005, 0.008, and 0.01; Iter = 20, 30, 40). In another two volunteers, variable amounts of k-space, i.e. 15%, 12%, 9%, and 6%, were acquired using the same λ (0.003) and Iter (20) to explore the limit of acceleration. In all 7 healthy volunteers, paired comparison between CS IR-SPACE (15% of k-space, λ of 0.003, and Iter of 20) and IR-SPACE was performed with respect to white-gray matter CNR, wall SNR, and wall-CSF CNR measured at the internal carotid artery supraclinoid segment. In addition, the 5 patients underwent both CS IR-SPACE and IR-SPACE scans that were respectively reviewed by a neuroradiologist.
The major imaging parameters for 3D acquisitions were: sagittal orientation; TR/TE 900/15 ms; isotropic spatial resolution 0.55 mm; number of slices 256 (CS IR-SPACE) and 224 (IR-SPACE); ETL 52; scan time 4min46sec (15% CS IR-SPACE) and 8min09sec (IR-SPACE). Moreover, the T1/T2 used for calculating refocusing flip angle series were prescribed at 1100/170 ms to help generate more signal in IVW [4].
RESULTS
With 15% of k-space data, λ exhibited a considerable impact on modulation of overall image quality in CS IR-SPACE imaging. A λ between 0.001 and 0.005 yielded consistently acceptable to good quality of vessel wall delineation, as shown in Fig. 1. In contrast, Iter had essentially no effect on image quality and wall delineation when changing from 20 to 40 (Fig. 2). A combination of λ = 0.003 and Iter = 20 appeared to be consistently good for the scenario of sampling 15% of k-space data in all 7 healthy subjects when visually compared with regular IR-SPACE. CS IR-SPACE images exhibited slightly higher white-gray matter CNR, wall SNR, and wall-CSF CNR (Fig. 3). Further attempts in acquiring even less k-space data showed dramatic deterioration in image quality, although the used λ and Iter were likely suboptimal for 6, 9, and 12% (Fig. 4). Two of the 5 patients were diagnosed of having IVW pathologies based on each of IVW scans alone. CS IR-SPACE and IR-SPACE showed comparable lesion delineation quality despite markedly different scan times (Fig. 5).DISCUSSION
In this study we proposed a 5-min whole-brain IVW imaging protocol using compressed sensing-based IR SPACE. Our preliminary study identified the optimal parameters, λ and Iter, that are image quality relevant for the scenario of sampling 15% of k-space data. It is likely that the scan time of CS IR-SPACE can be further pushed with additional optimization of those parameters. Faster IR SPACE imaging would help improve successful rate of IVW MRI and promote adoption in clinical practice, although potential sacrifice in image quality and delineation of wall pathologies await a systematic comparison study on a large patient cohort.Acknowledgements
This work was supported in part by American Heart Association (15SDG25710441) and National Institutes of Health (NHLBI 2R01HL096119)References
[1] Qiao Y et al. JMRI 2011;34:22. [2] van der Kolk AG et al. Eur Radiol 2013;23:2996. [3] Fan Z et al. MRM 2016;Feb 28. [4] Fan Z et al. ISMRM 2016;1446. [5] Stalder et al. MRM 2015;74:1652.Figures