3118
Three-dimensional black-blood multi-contrast protocol for carotid imaging using compressed sensing: a repeatability study1Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 2GE Healthcare, Amersham, United Kingdom, 3GE Healthcare, Waukesha, Wisconsin, United States, 4Department of Radiology, Cambridge University Hospitals NHS Foundation Trust, Cambridge, United Kingdom
Synopsis
Multi-contrast black-blood MRI protocol has demonstrated the ability to assess carotid plaque vulnerability. Current limitation for its wide application is the long scanning time. The purpose of this work is to evaluate the repeatability of a compressed sensing (CS) accelerated multi-contrast protocol at 3T.
Purpose
The purpose of this current work is to evaluate the repeatability of a compressed sensing (CS) accelerated multi-contrast protocol for black-blood carotid artery imaging at 3T.Material and methods
Study subjects: This study had ethical approval and informed consent was obtained from each volunteer and patient. Twelve volunteers (eight men, mean age 34, range: 24-55 years) and eight patients (four men, mean age 75, range: 72-87 years) with a carotid artery stenosis higher than 50% on duplex ultrasound were scanned at a 3T MRI scanner (MR 750, GE Healthcare, Waukesha, WI), using a four channel phased-array neck coil (PACC, MachNet, Roden, The Netherlands). To evaluate the interscan reproducibility of the sequences, the twelve volunteers were scanned for a second time using the same protocol. The average time interval between the two scans was 14 days [range 7 to 28 days].
CS: The compressed sensing was achieved by using a Gaussian pseudo-random distribution under-sampling pattern in k-space. A 32×32 region in the center of k-space remained fully sampled. In the image reconstruction, the following objective function was used, $$$\widehat{m}=\parallel\psi m\parallel _{1}$$$ such that $$$\parallel F\widehat{m}-y\parallel _{2}^{2}\leq\epsilon$$$, where $$$\psi$$$ is the sparsifying transform implemented as the nearest neighbor finite difference of the complex image m, F is the Fourier transform operator and y is the acquired k-space data1. Fifteen iteration loops were used to minimise the penalty (L1-norm). Details of image acquisition and reconstruction can be found in reference2.
Imaging protocol: The multi-contrast protocol was shown in Table 1. Coronal imaging slabs for the 3D sequences were centred at the carotid bifurcation. T1w images were acquired by a DANTE prepared3 3D fast spin echo (FSE) sequence without CS, and with CS acceleration factors of 1.5 and 2.0. T2w and PDw images were acquired using an iMSDE prepared4 3D FSE sequence. The iMSDE first-order moment was set to 412mTms2/m. A CS acceleration factor of 1.5 and ARC-based5 parallel imaging of phase×slice = 2×1 was used. CS and ARC were combined sequentially2. The acquired resolution for T1w, T2w and PDw was 0.6×0.6×1.4mm3.
Image analysis: Carotid artery contours from multi-contrast sequences were defined by an experienced reviewer using OsiriX (OsiriX 5.5.2, Pixmeo, Geneva, Switzerland). Mean values of lumen area, wall area and thickness were calculated per-vessel, per-subject and per-scan. Interclass correlations (ICCs) were calculated to evaluate the agreement of wall/lumen area and wall thickness measurements between two scans. For the patient scans, the intra-observer reproducibility was tested on the morphological T1w images. To test the inter-reader reproducibility, a second reviewer analysed the images and the results were compared with the first reviewer. Both reviewers made the measurements independently and were blinded to scanning parameters.
Results
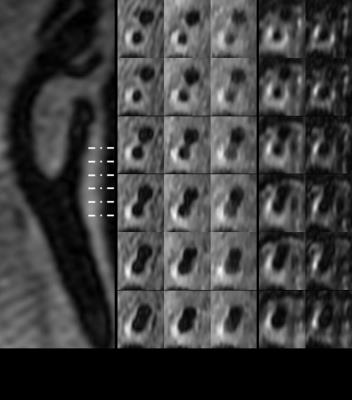
All twelve volunteers completed the repeated scans. Seven out of eight patients completed the scan. The ICCs for lumen and wall area at repeated scans with CS factor of 1.5 were given at Table 2. Good correlations were found between two scans (all ICCs >0.80). An example of a patient image is shown in Figure 1. The patients had a thicker vessel wall compared with volunteers (2.43±0.57mm vs. 1.16±0.21mm, p<0.05). There was no difference in measuring the wall thickness when using CS acceleration compared with non-CS acceleration (Non-CS vs. CS1.5: 2.43±0.57mm vs. 2.58±0.87mm, p = 0.58; Non-CS vs. CS2.0: 2.43±0.57mm vs. 2.39±0.85mm, p=0.59). Table 3 and 4 show intra-/inter-reader reproducibility of the patient images. All of the measurements had high intra-/inter-reader reproducibility (all ICCs>0.80).Conclusion
The results show the black-blood multi-contrast protocol could be accelerated by CS with adequate image quality and accurate morphology measurements. Future protocol design could take CS into consideration to reduce the total scanning time.Acknowledgements
We acknowledge funding from NIHR BRC and Addenbrooke's Charitable Trust.References
1. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magnetic resonance in medicine. 2007 Dec 1;58(6):1182-95.
2. King K, Xu D, Brau AC, Lai P, Beatty PJ, Marinelli L. A new combination of compressed sensing and data driven parallel imaging. InProceedings of 18th Annual Meeting of ISMRM, Stockholm, Sweden 2010 (p. 4881).
3. Li L, Miller KL, Jezzard P. DANTE: prepared pulse trains: A novel approach to motion-sensitized and motion-suppressed quantitative magnetic resonance imaging. Magnetic resonance in medicine. 2012 Nov 1;68(5):1423-38.
4. Wang J, Yarnykh VL, Yuan C. Enhanced image quality in black-blood MRI using the improved motion-sensitized driven-equilibrium (iMSDE) sequence. Journal of Magnetic Resonance Imaging. 2010 May 1;31(5):1256-63.
5. Beatty PJ, Brau AC, Chang S, Joshi SM, Michelich CR, Bayram E, Nelson TE, Herfkens RJ, Brittain JH. A method for autocalibrating 2-D accelerated volumetric parallel imaging with clinically practical reconstruction times. In Proc Intl Soc Magn Res Med 2007 (p. 1749).
Figures