3117
Estimation of motion-corrupted data using parallel imaging for carotid artery vessel wall imaging1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3FMRIB Centre, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 4Oxford Centre for Clinical Magnetic Resonance Research, Division of Cardiovascular Medicine, University of Oxford, Oxford, United Kingdom, 5Section on Magnetic Resonance Spectroscopy, National Institute of Mental Health, Bethesda, MD, United States
Synopsis
Carotid artery imaging is hampered by motion artefacts, including those caused by occasional swallowing motion. In this work, we extend previous work on intelligent reacquisition to estimate and replace motion-corrupted data. ‘Bad’ phase-encode lines were synthesized from surrounding ‘good’ lines using parallel imaging techniques. Estimation and replacement of corrupted data reduced background ghosting levels in comparison to the previous reacquisition approach. A small number of reacquisitions was maintained to ensure good quality data at the centre of k-space because these are essential for image quality and for calibration of the parallel imaging-based estimation.
Purpose
To reduce motion artifacts in carotid artery vessel wall imaging by combining reacquisition with estimation and replacement of motion-corrupted data.Introduction
Carotid artery vessel wall imaging sequences have high sensitivity to motion-corruption. Swallowing motion can cause severe artefacts, the manifestations of which depend on the nature of the motion and the timing relative to the k-space acquisition schedule. Previous approaches to mitigating swallowing artifacts include 1D tracking of the epiglottis, self-gating, and reacquisition with free induction decay navigators1-3. Here, previous work on the reacquisition approach4 is extended to attempt to estimate corrupted data using parallel imaging techniques rather than simply by reacquiring it. The redundancy of information provided by the multiple elements in an array receiver coil can be used to estimate ‘bad’ k-space lines from surrounding ‘good’ k-space lines. The estimation of corrupted data (ECD) strategy is combined with reacquisition of central k-space data, since these are essential for image quality and for calibration of the parallel imaging-based estimation.
The technique was implemented in a multiple spin-echo (MSE) sequence used for quantitative T2 mapping of the carotid artery by fitting T2 from 14 echoes5. In the current multi-slice implementation, phase-encode lines are acquired sequentially with a 2s repetition time (TR) between lines. This acquisition scheme is sensitive to motion during and between phase-encode lines, which generates inconsistencies in k-space leading to ghosting and blurring in the reconstructed images.
Methods
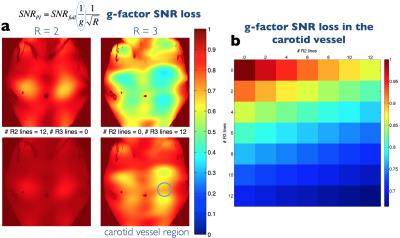
A ky=0 navigator acquisition and motion-scoring analysis framework described in previous work4 was used to identify phase-encode lines affected by motion. The chosen strategy was to estimate corrupted phase-encode lines and use 5 reacquisition TRs (10s) to ensure uncorrupted data at the centre of k-space, which were used to calibrate the parallel imaging algorithms. Using the terminology of conventional parallel imaging, single and pairs of corrupted phase-encode lines were termed R=2 and R=3 GRAPPA, respectively (see Fig. 1). G-factor related SNR loss was calculated6 and GRAPPA7 versus SPIRiT8 synthesis of the ‘missing’ lines was compared. A custom-built neck coil (PulseTeq, Surrey, UK) with 10 receive channels was used.
Nine healthy volunteers were scanned under a technical development ethics agreement on a Siemens Verio 3T. The following acquisition parameters were used: 14 echo-times ranging from 9.1 to 127.4ms at 9.1ms intervals, TR=2s, FOV=128×128mm2, matrix size=192×192, 5/8 partial Fourier and five 2mm slices (100% slice gap). A 60ms DANTE preparation module was used for flowing spin suppression9 with the following parameters: slice gradient amplitude 18mT/m, 120 pulses, flip angle 8°, 500μs between RF pulses, 340μs gradient duration. Images were reconstructed offline in Matlab (Mathworks).
Results
Figure 2 shows how the g-factor SNR loss associated with GRAPPA estimation of lines can be kept to acceptable levels by limiting the number of estimated lines, in particular by limiting the number of R=3 corrections.
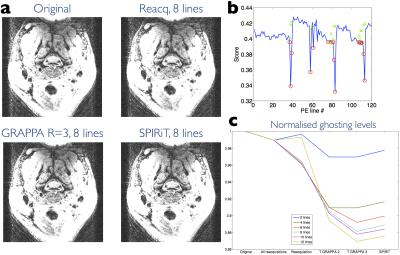
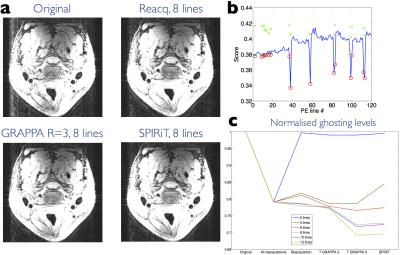
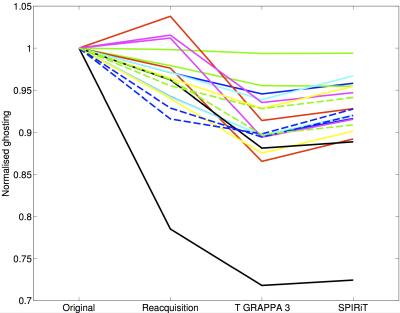
Image quality and ghosting levels are compared in Figs 3 and 4. Figure 4 demonstrates the substantial improvement offered by reacquisition and estimation when motion occurs during acquisition of lines close to the k-space centre. A summary over all 9 subjects of ghosting levels is provided in Fig. 5. The number of lines replaced was chosen on a scan-specific basis using the motion scores. GRAPPA estimation consistently offers the lowest ghosting levels.
Discussion
The proposed method appears to reduce ghosting levels further than by simply replacing motion-corrupted data with reacquisitions. Using surrounding data to synthesize phase-encode lines potentially offers better self-consistency than using reacquisitions from the end of a scan, by which time more motion could have occurred making data less consistent. Figure 5 demonstrates that using reacquisitions causes unpredictable increases in ghosting in some cases. Estimating data could also reduce time required for reacquisitions at the end of the scan, since here only a few central lines are needed to ensure adequate performance of the GRAPPA correction.
Alternative k-space acquisition schedules could be explored to reduce the number of R=3 (or greater) line corrections required, which are costly in terms of g-factor SNR loss. For example, a randomized ordering of phase-encode lines could potentially ‘spread’ the motion effects, minimizing the likelihood of corruption in contiguous lines.
The reacquisition and estimation techniques rely on the assumption that the subject returns to the original position after moving. Prospective motion techniques that can be used for neuroimaging are challenging in this setting due to the non-rigid body motion of the neck.
Conclusion
Estimating and replacing motion-corrupted data appears to be a promising method to reduce artefacts caused by occasional motion. The technique has been demonstrated in a vessel wall imaging setting which is hampered by swallowing motion.Acknowledgements
We are grateful to the National Institute for Health Research Oxford Biomedical Research Centre and for the facilities provided by the Acute Vascular Imaging Centre. We thank Andre van der Kouwe and Dylan Tisdall for code which the reacquisition scheme was based on. We are also grateful to Juliet Semple and Peter Manley for assistance with data acquisition.References
1. Koktzoglou I, Li D. Submillimeter isotropic resolution carotid wall MRI with swallowing compensation: imaging results and semiautomated wall morphometry. J Magn Reson Imaging 2007;25:815–823.
2. Fan Z, Zuehlsdorff S, Liu X, Li D. Prospective self-gating for swallowing motion: a feasibility study in carotid artery wall MRI using three-dimensional variable-flip-angle turbo spin-echo. Magn Reson Med 2012;67:490–498.
3. Dyverfeldt P, Deshpande VS, Kober T, Krueger G, Saloner D. Reduction of motion artifacts in carotid MRI using free-induction decay navigators. Journal of Magnetic Resonance Imaging 2014;40:214–220.
4. Frost R, Hess AT, Li L, Robson MD, Biasiolli L, Jezzard P. Selective reacquisition for motion artifact reduction in quantitative T2 mapping of carotid artery vessel wall. In Proceedings of the 24th Annual Meeting of ISMRM, Singapore, 2016 (abstract 2670).
5. Biasiolli L, Lindsay AC, Chai JT, Choudhury RP, Robson MD. In-vivo quantitative T2 mapping of carotid arteries in atherosclerotic patients: segmentation and T2 measurement of plaque components. J Cardiovasc Magn Reson 2013;15:69.
6. Breuer FA, Kannengiesser SAR, Blaimer M, Seiberlich N, Jakob PM, and Griswold MA. General formulation for quantitative G-factor calculation in GRAPPA reconstructions. Magn Reson Med, 2009;62:739–746.
7. Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, and Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med, 2002;47:1202– 1210.
8. Lustig M and Pauly JM. SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space. Magn Reson Med, 2010;64:457–471.
9. Li L, Chai JT, Biasiolli L, Robson MD, Choudhury RP, Handa AI, Near J, Jezzard P. Black-Blood Multicontrast Imaging of Carotid Arteries with DANTE-prepared 2D and 3D MR Imaging. Radiology 2014;273:560–569.
Figures