3116
Dynamic Nitroxide-Enhanced MRI Detects Oxidative Stress in the Hearts of Mice Subject to Angiotensin II Infusion1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Radiology, University of Virginia, Charlottesville, VA, United States
Synopsis
Oxidative stress contributes importantly to the pathophysiology of many types of cardiovascular disease. Nitroxides are relatively stable free radicals that have been used as redox-sensitive MRI contrast agents in preclinical studies to assess tumor redox status. We implemented a dynamic nitroxide-enhanced MRI method to test the hypothesis that MRI can detect cardiac oxidative stress in vivo. Imaging was performed in untreated controls and mice infused with angiotensin II for 7 days. The MRI signal decay rate in the heart was significantly higher in the angiotensin II group, indicating that these methods detect cardiac oxidative stress due to angiotensin II infusion.
Introduction
Oxidative stress, defined as an excess production of reactive oxygen species (ROS) relative to antioxidant defenses, has been shown to play an important role in the pathophysiology of a wide array of cardiovascular diseases [1, 2]. While techniques to estimate oxidative stress through biomarkers in circulation and serum have been developed and widely applied [3], there are no established commonly-available methods to noninvasively assess oxidative stress localized to the heart. Nitroxide stable free radicals can serve as T1-shortening contrast agents that lose their T1-shortening property as they undergo in vivo reduction reactions. Given this property, nitroxides have been used as redox-sensitive MRI contrast agents in preclinical cancer imaging to assess tumor redox status [4, 5]. We tested the hypothesis that dynamic nitroxide-enhanced MRI can detect oxidative stress in the heart. Specifically, we studied untreated control mice and mice infused with angiotensin II for 7 days, a well-established method of inducing oxidative stress in the cardiovascular system [6]. We hypothesized that dynamic nitroxide-enhanced MRI would detect an increased decay rate of the nitroxide-enhanced MRI signal, indicative of elevated cardiac oxidative stress in mice after angiotensin II infusion.Methods
The nitroxide contrast agent 3-Carbamoyl-PROXYL (3CP) (Sigma–Aldrich, St. Louis, MO) was chosen because it is water soluble, commercially available, well tolerated by mice, and has relaxation rates R1 (or 1/T1) that increase linearly with concentration in the range of 0.5 to 35 mM. The relaxivity of 3CP in saline solution at 7T was previously measured to be 0.139 mM-1sec-1. Angiotensin II infusion was administered by implanting mice with Alzet micro-osmotic pumps (Model 1002; Durect Corp; Cupertino, CA) loaded with angiotensin II at the concentration necessary to provide an infusion rate of 0.7 mg/kg body weight per day, as previously described [6]. Wild type male C57Bl/6 mice with angiotensin II infusion for 7 days (n = 7) and untreated controls (n = 6) underwent MRI studies using a 7T system (Clinscan, Bruker). The electrocardiogram (ECG), body temperature, and respiration were monitored during imaging using an MR-compatible system (SA Instruments, Stony Brook, NY). During MRI, mice were anesthetized with 1.25% isoflurane and maintained at 36 ± 0.5°C using circulating warm water. After localizer imaging, T1-weighted dynamic contrast enhanced MRI was performed in a mid-ventricular short-axis slice before and consecutively after injection for 10 minutes. 3CP was administered through an indwelling tail vein catheter at 2 mmol/kg body weight over 3 to 4 seconds. The concentration of 3CP in the bolus solution was 50 mg/mL. ECG-gated saturation-recovery rapid gradient echo imaging was used with: field of view = 34 x 24 mm2, matrix size = 128 x 102, TE/TR = 1/1.8 ms, flip angle = 15 degrees, slice thickness = 1 mm, saturation delay = 45 ms, number of segments = 15 and number of averages = 16, with a total scan time of approximately 30 seconds per image. Signal intensities of blood and myocardial regions were normalized by proton density images and were converted to 3CP concentrations using the methods described by Cernicanu and Axel [7]. The concentration vs. time relationship was modeled as an exponential decay, and the decay rate k was calculated by least-squares fitting of the average myocardial nitroxide concentration with the following equation: ln(3CP concentration) = k * time + constant.Results
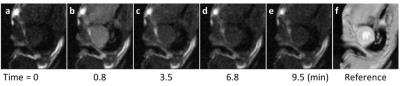
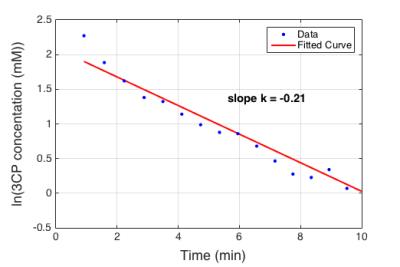
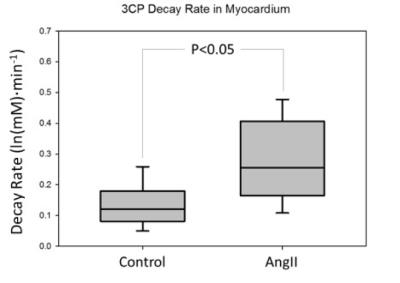
Figure 1 shows example dynamic nitroxide-enhanced MR images of a mouse heart obtained before and after injection of 3CP, showing a decrease in signal over time as 3CP decays. Figure 2 shows an example ln([3CP]) vs. time curve obtained from a mouse along with the linear fit. The decay rate in the example shown was: k = 0.21 ln(mM)*min-1. Figure 3 summarizes data from all the mice and shows that the 3CP-enhanced signal decay rate is statistically greater in angiotensin II infused mice (0.28 ± 0.05 ln(mM)*min-1) compared to untreated controls (0.13 ± 0.03 ln(mM)*min-1).Conclusion and Discussion
Redox reactions between the paramagnetic nitroxide, intracellular antioxidants, and intracellular oxidizing species contribute to the decay of paramagnetic nitroxides. The elevated decay rate in the angiotensin II infusion group compared to the untreated group suggests that dynamic nitroxide-enhanced MRI can detect increased cardiac oxidative stress due to angiotensin II infusion. These methods may be broadly applicable to preclinical studies of mechanisms and experimental therapies in numerous models of cardiovascular disease. Nitroxide-enhanced MRI also shows potential for translation to humans as safety profiles appear promising and some of these compounds have already been tested in clinical trials applied in the skin and eyes.Acknowledgements
Funding: NIH R01 EB001763 and AHA pre-doctoral fellowship 16PRE29750008.References
1. Tsutsui, H., S. Kinugawa, and S. Matsushima, Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol, 2011. 301(6): p. H2181-90.
2. Dhalla, N.S., R.M. Temsah, and T. Netticadan, Role of oxidative stress in cardiovascular diseases. J Hypertens, 2000. 18(6): p. 655-73.
3. Ho, E., et al., Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol, 2013. 1: p. 483-91.
4. Hyodo, F., et al., Probing the intracellular redox status of tumors with magnetic resonance imaging and redox-sensitive contrast agents. Cancer Res, 2006. 66(20): p. 9921-8.
5. Matsumoto, K., et al., High-resolution mapping of tumor redox status by magnetic resonance imaging using nitroxides as redox-sensitive contrast agents. Clin Cancer Res, 2006. 12(8): p. 2455-62.
6. Landmesser, U., et al., Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension, 2002. 40(4): p. 511-5.
7. Cernicanu, A. and L. Axel, Theory-based signal calibration with single-point T1 measurements for first-pass quantitative perfusion MRI studies. Acad Radiol, 2006. 13(6): p. 686-93.
Figures