3100
3D high resolution imaging of human hearts for visualization of the cardiac structure1IHU LIRYC/U1045, Université de Bordeaux, Pessac, France, 2Mathematical Cell Physiology, Max Delbrück Center for Molecular Medicine, berlin, Germany, 3Auckland Bioengineering Institute, University of Auckland, Auckland, New Zealand, 4IHU LIRYC/U1045, CHU Bordeaux, Pessac, France
Synopsis
The motivation of this study is to develop high resolution 3D MR imaging of human hearts to characterize the cardiac structure non-invasively. For this purpose, T1-weighted images and diffusion MRI in 3D from intact and infarcted hearts were acquired and analyzed to compare myocyte and myolaminar orientations in healthy and pathological regions.
Purpose
High Resolution Magnetic Resonance Imaging (HR-MRI) can provide information on myocardial fiber orientation, and myolaminar/sheetlet structure. Myocyte and myolaminar orientations influence cardiac electromechanical properties and are essential for electromechanical modeling1. It has been demonstrated in hearts from rats2, 3 and sheep4 that HR-MRI and diffusion MRI can be used to describe this structural information at high field MRI on ex vivo samples. In this study, we applied these approaches to whole ex vivo human hearts from patients not eligible to cardiac transplantation. 3D T1-weighted(T1-w) and diffusion images were acquired at a spatial resolution of 200 µm or better using a 9.4T/30 cm magnet and a dedicated transit/receive MR coil designed for this application.Methods
Sample preparation: The samples (N=2) were obtained through the CADeNCE (led by Pr. M. Haïssaguerre) project approved by the French Biomedicine Agency. A healthy heart (53 years old, female) and a pathological heart (56 years old, male) were flushed with cold cardioplegic solution before surgery and then perfusion-fixed for at least 6h with 4% formaldehyde in PBS containing 2 ml/L Dotarem (gadoterate meglumine, Guerbet, France). Imaging was carried out with the heart removed from formaldehyde solution and immersed in fomblin oil to reduce susceptibility artifacts.
MR acquisition: All experiments were performed at 9.4T/30cm (Bruker Biospin MRI, Ettlingen Germany). A cylindrical (165 mm inner diameter) 7 channels volume array Tx/Rx was used for ex vivo imaging. A 3D T1w FLASH sequence was run to image the whole healthy heart volume, using TE/TR=12/30 ms; matrix-size=800×666×512; voxel dimensions= 150×150×234μm3; flip angle= 33°, 25 averages for a total acquisition-time of 36hr48min; for the pathologic heart acquisition parameters were TE/TR=13/30ms; matrix-size=1000×830×512; voxel dimensions= 120×120×215μm3; flip angle = 25°, 35 averages for a total acquisition-time of 63hr45min. DT-MRI was carried out with a set of 6 directions using a 3D diffusion-weighted spin-echo sequence with TE = 15ms, TR= 500ms, at an isotropic resolution of 600 μm.
Post processing: Analysis of the data was performed with the Visualization Toolkit libraries. Low and high cutoff thresholds were applied to the fraction anisotropy and to the T1-w image to define a binary mask. For each voxel in the segmented datasets, elevation angles (helix and transverse angles) were computed from primary eigenvector, as described in references1, 2.
Results
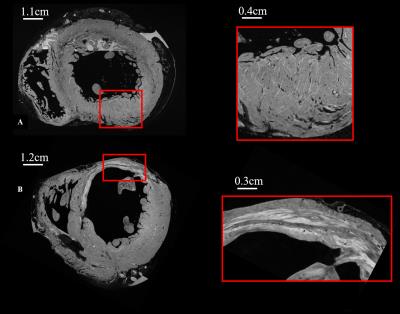
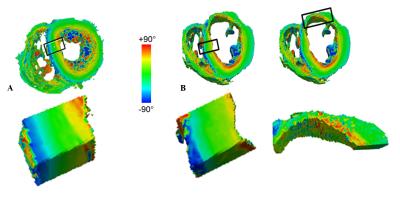
Figure 1-A presents HR T1-w images of the control heart. A zoom in the left ventricle (LV) free wall (Figure 1-A) shows the details of the sheetlet structure in the myocardium. Images from the pathological heart (figure 1-B) highlight a wall shrinkage in the infarct region (red box in Fig1-B) which extends to the interventricular septum. A reorganization with different layers and a remodeling of the tissue inside the infarct can be observed in the region of interest (ROI). LV thickness in the infarct region was decreased by 50% compared to the safe area (thickness infarct: 5.9 ±0.5 mm and safe area= 12.6±0.4 mm). In the healthy heart, the LV thickness appears homogeneous with a size of 11.1±0.8 mm. Helix angles for both samples are represented in figure 2-A and B. No spatial filtering were applied, raw data diffusion were highly spatially consistent in the healthy heart. A septal LV ROI is shown for the healthy and pathological hearts, with the same helical transmural profile in this healthy area for both hearts, displaying a smooth transition from a negative helix in the epicardium to a positive helix in the endocardium. On the contrary, myocyte structure appears more disorganized in the infarcted region.Discussion
HR-MRI images provide valuable information in healthy and pathologic human tissues in 3D to characterize myocytes and myolaminar structure. Quantitative analysis of the helix angle through transmural wall requires accurate registration of samples to obtain robust comparison between subjects. Such perspectives are currently under evaluation. All these results will be coupled with electrical measurements and histology data to validate orientation of fibers or reorganization of muscle with collagen.Conclusion
High resolution 3D MR imaging allows visualization of the cardiac architecture. Combining structural and electrical data may help to better understand the links between arrhythmia and structural changes of the myocardial structure. These data may also be used to create an atlas of images of heart diseases and will be incorporated into computer simulations for better modelling of electrical propagation.Acknowledgements
All researchers involved in the CADeNCE (fibrillation CArdiaque et DyssyNChronie Electrique du coeur) project are gratefully acknowledge for their valuable contributions.References
1- LeGrice IJ, Pope AJ, Sands GB, et al. Progression of myocardial remodeling and mechanical dysfunction in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2012;303:H1353–65.
2- Bernus O, Radjenovic A, Trew ML, et al. Comparison of diffusion tensor imaging by cardiovascular magnetic resonance and gadolinium enhanced 3D image intensity approaches to investigation of structural anisotropy in explanted rat hearts. J Cardiovasc Magn Reson. 2015;17:31.
3- Gilbert SH, Sands GB, LeGrice IJ, et al. A framework for myoarchitecture analysis of high resolution cardiac MRI and comparison with diffusion tensor MRI. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:4063-6
4- Gilbert SH, et al. Measurement and quantification of sheep cardiac myocyte and sheetlet orientation from high-field 80 × 80 × 160 μm contrast enhanced T1W MRI. 2015. ISMRM abstractnumber:2259.
Figures