3094
Hyperpolarized [1-13C]-MRI in an ectothermic reptile1Section for Zoophysiology (Department of Bioscience), Aarhus University, Aarhus C, Denmark, 2Comparative Medicine Lab (Department of Clinical Medicine), Aarhus University, Aarhus N, Denmark, 3MR Research Centre (Department of Clinical Medicine), Aarhus University, Aarhus N, Denmark, 4Danish Diabetes Academy, Odense, Denmark
Synopsis
Many non-mammalian vertebrates hold enormous potential as “model animals” for various fields of basic physiological and biomedical research. Hyperpolarized magnetic resonance imaging (MRI) can provide quantitative in vivo information about metabolic processes including major pathways of the citric acid cycle and glycolysis via spectral differences of pyruvate intermediates. The combination of [1-13C]-MRI and model animals exhibiting “selected physiological traits” may be a strong tool for gaining novel insights into relevant metabolic mechanisms. In this pilot study we test, for the first time, the application of [1-13C]-MRI in an ectothermic reptile.
Background and purpose
Non-mammalian vertebrates hold enormous potential as models that may be used in diverse fields of basic physiological and biomedical research. Following the August Krogh principle, “For many problems there is an animal on which it can be most conveniently studied”1,2, snakes, particularly pythons, have been extensively studied due to a marked up-regulation of digestive processes in response to feeding after prolonged fasting3, including pronounced phenotypic flexibility of organs4. Such “exotic” models, however, remain largely neglected in studies using non-invasive imaging techniques that allow measurements of dynamic metabolic processes. A significant challenge in this endeavour may be their typically low metabolic rates compared to the mammals utilized in biomedical research.
Hyperpolarized [1-13C]Pyruvate magnetic resonance imaging (MRI) allows for detailed studies of various metabolic and physiological processes in vivo. Earlier, our group evaluated hyperpolarized MRI in an amphibian, the axolotl (Ambystoma mexicanum). We now present preliminary results using [1-13C]Pyruvate MRI in a reptile, the ball python (Python regius), at two temperatures.
Methods
One juvenile python (male; body mass: 290 g; snout-vent length 74 cm), housed at Aarhus University was used. Food was withheld for three weeks before the experiments began. Two days prior to MRI a PE50 vascular catheter was inserted in the jugular-vein under general anaesthesia with 2-5% Isoflurane (IsoFlo vet 100%, Abbott, UK), the surgical procedure lasted <45 min.
For MRI the animal was anaesthetized via intra-venous (IV) injection of propofol (10 mg/ml propolipid, Fresenius Kabi, Sweden); induction dose was 10 mg/kg and a maintenance dose of 3 mg/kg was administered every 20 min. The snake was manually ventilated with air, receiving 4×12.5 mL every 5 min. A 60 sec [1-13C]Pyruvate-MR acquisition was initiated 20 s following a bolus IV-injection of 1 mL [1-13C]Pyruvate. The python was imaged at room temperature of 21.4 °C and again following heating to 24.1 °C using an insulated electrical heating blanket.
A series of 13C IDEAL spiral scans was acquired using following parameters; acquiring images every 5 s with a flip angle of 10º, 11 IDEAL echoes and one initial spectrum per IDEAL encoding, TR/TE/ΔTE=100 ms/0.9 ms/0.9 ms, FOV = 200. Then, T2 FLAIR weighted anatomical [1H]-MR images was acquired using following parameters; TE 155 ms, TR 8652 ms, averages 2, inversion time 2250 ms, flip angle 90, FOV 320.
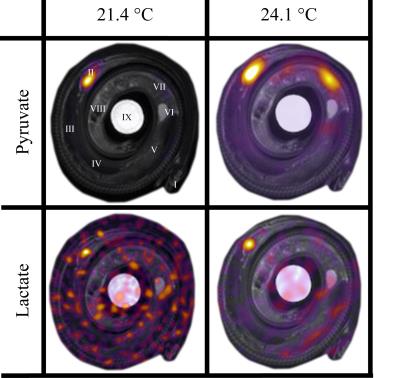
Temperature and heart rate (HR) were monitored before and after MR imaging. Osirix software (Pixmeo, Geneva, Switzerland) was used to perform region of interest (ROI) analysis of [1-13C]Pyruvate data and the formation of images using window width and level settings to optimally visualize the distribution of [1-13C]Pyruvate overlaying T2 weighted morphological images (figure 1).
Results and discussion
Heart rate at 21.4 °C was 20 min-1 and increased to 28 min-1 at 24.1 °C. The in vivo distribution of pyruvate and lactate is displayed in Figure 1, with the ROI-analysis of metabolite (lactate, alanine, bicarbonate and pyruvate-hydrate) to pyruvate ratios reported in table 1.
The injected pyruvate was clearly visible in the heart of the python at both temperatures (fig. 1). Further, pyruvate could be visually identified in the region of the small intestine and further as background signal in the entire body at 24.1 °C (fig. 1). Biochemical pyruvate into lactate conversion was clearly apparent in the heart at 24.1 °C, and although lactate is discernible in the heart also at 21.4 °C, the lactate signal at this cool situation appears primarily as background noise (fig. 1) and causes great variation in the metabolite:pyruvate rations of table 1. All additional metabolites displayed only background signal at both 21.4 °C and 24.1 °C (data not shown).
The peak signal of lactate was delayed compared to peak signal of pyruvate at both temperatures, but to the greatest extend in the coolest situation (data not shown). This observation reveals the ability of the methodology to identify in vivo metabolism of pyruvate in ectothermic animals experiencing an increase in overall metabolism with increasing body temperature. The maximal metabolite:pyruvate ratio for lactate, alanine and bicarbonate in the heart at 24.1 °C was 0.009, 0.008 and 0.004, respectively, with a percentage change of 11.9, -24.7 and -8.3, respectively, after heating from 21.4 °C to 24.1 °C (table 1).
Hyperpolarized MRI may provide spatial and temporal information of the in vivo conversion of selected biomolecules fundamental to biological function in all animals, but the application for non-mammalian animals remain largely unexplored. In this pilot study we have demonstrated that, despite the fast polarisation decay of hyperpolarized pyruvate (<60 sec), in vivo conversion of pyruvate to lactate can be identified in an ectothermic animal.
Acknowledgements
No acknowledgement found.References
1. Krebs HA. The August Krogh Principle: ‘for Many Problems There Is an Animal on Which It Can Be Most Conveniently Studied’. J Exp Zool. 1975;194(1):221-226.
2. Krogh A. The Progress of Physiology. Science 70 (1809). Science. 1929:200–204.
3. Riquelme CA, Magida JA, Harrison BC et al. Fatty acids identified in the Burmese Python promote beneficial cardiac growth. Science. 2011; 334 (6055): 528–531.
4. Wang T, Hung CCY, and Randall DJ. The comparative physiology of food deprivation: From feast to famine.” Annu Rev Physiol. 2006;68(1): 223–51.
Figures