3093
Enhancing metabolic imaging of energy metabolism in traumatic brain injury using hyperpolarized [1-13C]pyruvate and dichloroacetate1Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 2Anesthesiology and the Center for Shock, Trauma, and Anesthesiology Research, University of Maryland School of Medicine, Baltimore, MD, United States
Synopsis
We investigated the use of dichloroacetate (DCA) in 13C-pyruvate imaging of traumatic brain injury as a way to improve bicarbonate signal strength and to elucidate changes in pyruvate dehydrogenase activity. Rats were injured with a controlled cortical impact and then injected with 13C-pyruvate before and after administration of DCA. Spectrally-resolved imaging was performed on the brain to quantify the resulting pyruvate, lactate, and bicarbonate signals. The bicarbonate signal and bicarbonate-to-lactate ratio were found to be sensitive to traumatic brain injury, and were affected equally by DCA in injured and uninjured hemispheres of the brain.
Purpose
Traumatic brain injury (TBI) is a leading cause of death and disability in people under age 45 and can lead to cognitive impairments, mood disorders, and neurodegenerative diseases. We recently showed that magnetic resonance spectroscopy (MRS) of hyperpolarized 13C-pyruvate can provide a direct, non-invasive method for studying the effects of TBI on energy metabolism1. In particular, 13C-bicarbonate signal was found to be lower on the injured side of the brain, which indicates decreased pyruvate dehydrogenase (PDH) activity. This loss of activity could be due to cell death, enzyme dysfunction, or PDH inhibition by pyruvate dehydrogenase kinase (PDK). To further explore which mechanism is at play, we performed MRS experiments with hyperpolarized [1-13C]pyruvate following the administration of dichloroacetate (DCA), an inhibitor of PDK. By comparing PDH activity before and after DCA administration, the relative levels of PDH inhibition were determined.Methods
Traumatic brain injury was induced in the left parietal lobe of healthy adult male rats (5 Sprague Dawley and 1 Wistar, 233–256 g body weight) using a controlled cortical impact (CCI) device (Pittsburgh Precision Instruments, Pittsburgh, PA). After a left-sided craniotomy was performed, the 5-mm round impactor tip was accelerated to 5 m/s with a vertical deformation depth of 2.0 mm and an impact duration of 50 ms. At 3.5-4 hours post injury, hyperpolarized [1-13C]pyruvate imaging was performed using a clinical GE 750w 3T MR scanner (GE Healthcare, Waukesha, WI) and a doubly tuned (1H/13C) quadrature coil (∅ = 50 mm, USA Instruments Inc., Aurora, OH). The rats were anesthetized with 1.5-2% isoflurane in 1.0 L/min oxygen. They were injected in a tail vein with ~1.1 mmol/kg of ~140 mM [1-13C]pyruvate, which was hyperpolarized to ~50% liquid state polarization via dynamic nuclear polarization using a SpinLab polarizer (Research Circle Technology, Niskayuna, NY). Spectrally-resolved imaging of the brain was initiated 30 s after injection using a phase-encoded free-induction decay chemical shift imaging sequence with the following parameters: single axial (animal coronal) 8 mm slice centered on the injury, 40x40 mm2 field-of-view, 16x16 matrix, 5000 Hz spectral width, 19 s acquisition time. Pyruvate, lactate, alanine, and bicarbonate peaks were phased, baseline-corrected, and integrated to create a voxel-by-voxel map of relative metabolite concentrations. Comparisons were made between ROIs of the cerebral cortex ipsilateral and contralateral to the injury. As controls, 13C-pyruvate imaging was performed in the same way on healthy adult male rats that were uninjured (7 Sprague Dawley and 2 Wistar) or that underwent sham surgery (craniotomy but no CCI, 4 Sprague Dawley). For 2 control, 3 sham, and 3 CCI rats, the hyperpolarized imaging experiment was repeated 45 minutes after intravenous injection of DCA (200 mg/kg).Results
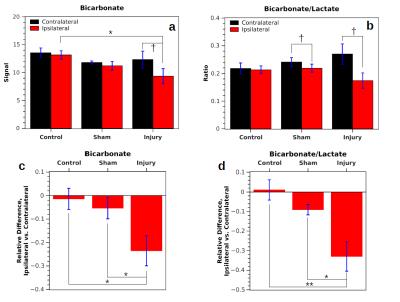
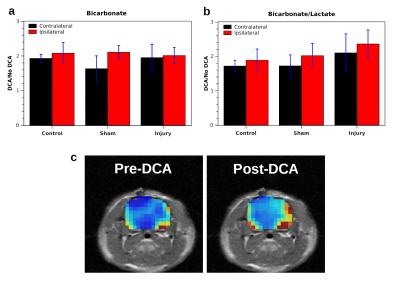
In measurements without DCA administration, both the bicarbonate signal (Bic) and the ratio of bicarbonate to lactate signal (Rbl = Bic/Lac) were significantly lower in the injured hemisphere following CCI (Fig. 1). Following injury, Rbl was 0.17±0.03 in the ipsilateral hemisphere and 0.27±0.04 in the contralateral hemisphere (p = 0.026, correlated t-test). In controls, no significant difference in Bic or Rbl existed between the two sides. Following DCA injection, the bicarbonate signal of the ROIs increased between 1.6 and 2.1 times and Rbl increased between 1.7 and 2.4 times, with no differences between the two sides reaching statistical significance (Fig. 2). The bicarbonate signal was significantly lower on the injured side of CCI rats following DCA administration.Discussion
Mitochondrial damage is known to occur following TBI and results in a disruption of oxidative phosphorylation specifically manifested as decreased activity of PDH complex, the enzyme complex that links glycolytic with oxidative metabolism by converting pyruvate to CO2 and acetyl-CoA2,3. As a result, there is a decrease in oxidative metabolism and a decrease in bicarbonate (formed from CO2) at the injury site. Our results following DCA administration suggest that the decreased PDH activity is due to cell death or enzyme dysfunction rather than reversible enzyme inhibition by PDK. The latter would have produced a greater increase in bicarbonate signal in the ipsilateral hemisphere following DCA administration. Instead, the equivalent signal increase suggests that PDH is inhibited equally by PDK in both hemispheres. Administration of DCA could be advantageous as a way to boost the signal-to-noise ratio of bicarbonate signal without skewing the relative signal levels in injured versus uninjured tissue.Conclusion
Hyperpolarized metabolic imaging with [1-13C]pyruvate detects a significant change in PDH metabolism following a CCI injury in rats, which is likely caused by cell death or dysfunction in the PDH enzyme rather than inhibition by PDK.Acknowledgements
This work was supported by NIH grant R21 NS096575.References
1. DeVience SJ, Lu X, Proctor J, et al. Metabolic imaging of energy metabolism in traumatic brain injury using hyperpolarized 13C-pyruvate. Proc 24th ISMRM annual meeting, Singapore, 2016, 669.
2. Robertson CL, Saraswati M, and Fiskum G. Mitochondrial dysfunction early after traumatic brain injury in immature rats. Journal of neurochemistry, 2007;101(5):1248-1257.
3. Sharma P, Benford B, Li ZZ, and Ling GS. Role of pyruvate dehydrogenase complex in traumatic brain injury and measurement of pyruvate dehydrogenase enzyme by dipstick test. Journal of emergencies, trauma, and shock, 2009;2(2):67-72.
Figures