Keita Saito1, Deepak Sail2, Burchelle N. Blackman2, Hellmut Merkle3, Rolf E. Swenson2, James B. Mitchell1, and Murali C. Krishna1
1National Cancer Institute, Bethesda, MD, United States, 2National Heart, Lung, and Blood Institute, 3National Institute of Neurological Disorder and Stroke
Synopsis
5,5-Dimethyl-1-pyrroline-N-oxide
(DMPO) is used to detect reactive oxygen species in vitro, and
N-acetyl-L-cysteine (NAC) is an antioxidant. We synthesized 13C-labeled
DMPO and NAC, and investigated feasibility of hyperpolarized 13C-DMPO
and 13C-NAC for evaluating oxidative stress in mice. Hyperpolarized 13C-DMPO and 13C-NAC
provided a single peak at 76 ppm and
174 ppm, respectively, and the T1 relaxation time was sufficiently
long to apply them for mouse imaging. The signals 13C-DMPO
and 13C-NAC were also detected
in a mouse body after intravenous injection. The results showed 13C-DMPO
and 13C-NAC can be applied
to some disease models to evaluate oxidative stress in vivo.
Introduction
Reactive oxygen species (ROS) such
as superoxide anion radical and hydroxyl radical play an important role in various
physiological processes, but on the other hand they readily react with
biomolecules and disrupt those functions. The excessive production of ROS is
harmful to living body, and considered to be related to various diseases. Therefore,
evaluation of oxidative stress in living body would be of help not only to
understand the mechanisms of the diseases but also to develop prophylactic and therapeutic
methods. 5,5-Dimethyl-1-pyrroline-N-oxide (DMPO) is a spin trap agent frequently
used to detect oxygen radicals in vitro with electron paramagnetic resonance
spectroscopy, and N-acetyl-L-cysteine (NAC) is an antioxidant clinically used. The
reaction of both compounds with ROS were well investigated in vitro studies,
but the pharmacokinetics, metabolism, and the reaction of them in vivo is not
elucidated in vivo. Recent development of 13C-MRI with hyperpolarized
13C-labeled compounds enabled us to detect 13C-labeled
compounds and those metabolites in vivo. In this study, we synthesized 13C-labeled
DMPO and NAC, and investigated feasibility of hyperpolarized 13C-DMPO
and 13C-NAC to evaluate oxidative stress in living animals.
Methods
30 μL
of [5-13C]DMPO-d9 containing
15 mM Finland-HCl or 100 μL of [1-13C]NAC
containing 15 mM OX063 was mixed with 1.5 μL
of 50 mM gadolinium chelate ProHance, polarized for 1-2 hour using a
hyperpolarizer (HyperSense, Oxford Instruments), and rapidly dissolved in 4.5
mL PBS containing 100 mg/L EDTA. The hyperpolarized [5-13C]DMPO-d9 (60 mM) or [1-13C]NAC (15 mM) was intravenously injected to a
mouse through a catheter placed in the tail vein of the mouse (12 μL/g body
weight). 13C-spectra in the mouse body were acquired every 1 sec
after the injection, with the flip angle of 10º. 13C-MRI studies were performed on a 3
T scanner (MR Solutions) using a 30 mm home-built 13C saddle coil.
Results
Non-labeled
DMPO and NAC were hyperpolarized, and 13C-MR spectra of them were
measured using the 3T scanner. Hyperpolarized DMPO and NAC provided a single
peak at 76 ppm and 174 ppm, respectively on the 13C-spectrum. We
synthesized [5-13C]DMPO-d9 and [1-13C]NAC. They were hyperpolarized
and dissolved in PBS to estimate the T1 relaxation time. The T1
relaxation time of [5-13C]DMPO-d9 and [1-13C]NAC was 60
sec and 18 sec, respectively in the 3 T magnet (Figure 1). Then, 13C-MR
spectroscopic measurements were carried out with mice. The solution of
hyperpolarized [5-13C]DMPO-d9 or [1-13C]NAC was
intravenously injected into the mouse, and 13C spectra in the mouse body
were acquired every 1 sec. The signal of [5-13C]DMPO-d9 was detected
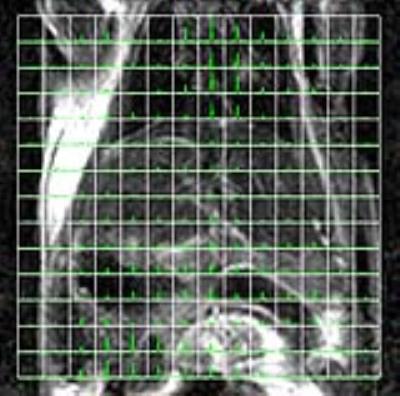
in the mouse body for more than 100 sec after the DMPO injection, and the signal was sufficiently large for imaging. 13C-chemical
shift imaging revealed that DMPO was distributed through the mouse body (Figure
2). The signal was higher in the chest and abdomen region, and signal from
liver region was relatively small compared to the other region. The signal of [1-13C]NAC
was also detected in the mouse body, but it was smaller than DMPO.
Conclusion
Hyperpolarized 13C-DMPO
provided sufficient magnitude of the 13C signal and long T1
to be applied to some disease models of mice. The signal of NAC was also detected
in mouse body, but the T1 was short compared to DMPO, and optimization
of the experimental conditions is necessary for further animal experiments.
Acknowledgements
This study was supported by
intramural research program of NCI/NIH.
References
No reference found.