3090
Assessing Gas Exchange via Co-Administration of Hyperpolarized [1-13C] Pyruvate and 13C-Bicarbonate1Radiology, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
We assess gas exchange in healthy and acutely injured rat lungs by measuring the difference between bicarbonate-to-pyruvate signal-ratio in the right vs. left ventricle of the heart following co-administration of hyperpolarized [1-13C] pyruvate and 13C-bicarbonate.
Introduction
Hyperpolarized (HP) [1-13C]-pyruvate and 13C-bicarbonate are often co-administered in preclinical imaging experiments to measure both pH and metabolic activity simultaneously [1-3]. As the HP 13C-bicarbonate transits through the lungs, it is in constant exchange with 13CO2, which is transported out of the lung capillary bed into the gaseous state, where the longitudinal relaxation rate is increased by several orders of magnitude. By the time the hyperpolarized bolus reaches the left ventricle, there should be significant decay in the bicarbonate signal in the presence of healthy gas exchange. By taking advantage of this process, we demonstrate that gas exchange measurements based on the difference in pyruvate-to-bicarbonate signal-ratio between the right and left ventricles of the heart can be used as an indicator of lung health.Methods
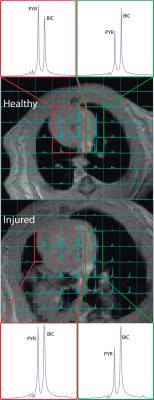
Nine Sprague Dawley rats were used for this study. Injury was created in N=5 rats using an acute lung injury model [1] to recreate the conditions of stomach acid aspiration; the injury is induced through tracheal acid aspiration (pH 1.25, 2.0mL/kg). A mixture of hyperpolarized [1-13C] pyruvate and 13C-bicarbonate was produced by rapidly decarboxylating pyruvate with hydrogen peroxide at elevated pH using the method described in our previous work [1]. The solution produced with this method is neutral and isotonic, and produces approximately 20mM each of pyruvate and bicarbonate/CO2. The solution was quickly injected through a tail vein catheter. Four injured animals and the healthy control animals were imaged using a 16x16 FID-CSI sequence (TR/TE = 25/0.38ms, SW = 6kHz, α=12°). The imaging was initiated 8 seconds after starting the injection of the hyperpolarized solution, in order to ensure that the majority of the HP compound had passed through the lung vasculature only once at the time of imaging. The fifth injured rat was imaged both before and after acid-aspiration injury to compare the difference in pyruvate-to-bicarbonate ratio between ventricles in the same animal. The spectroscopic data was processed using custom MATLAB routines. The right and left ventricles were manually segmented in the acquired carbon images based on their overlays on anatomical axial proton images. The pyruvate and bicarbonate signals in each segment were calculated by integrating under the corresponding peak in the average spectrum from all of the voxels in that segment. Statistical analysis of the data was preformed in R.Results and Discussion
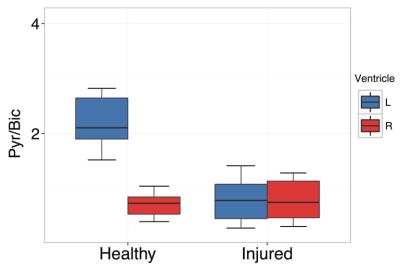
In healthy subjects, the pyruvate-to-bicarbonate ratio in the left ventricle of the heart was found to almost triple that within the right ventricle, indicating that a significant proportion of the HP bicarbonate in lung vasculature is exchanged with CO2 and lost to the gas phase during a single transit through the lungs. By comparison, injured subjects were found to have approximately equal pyruvate to bicarbonate ratios in both ventricles, likely indicating compromised gas exchange, with very little CO2 lost to the gas phase. The same result is seen in the injured subject for which pre- and post-injury carbon-13 images were obtained, suggesting that the results are not due to differences among individual subjects. The quantitative rigor of this method is currently limited due to the variability of both in-vivo 13C-bicarbonate and in-vivo [1-13C]-pyruvate T1 measurements [4-5], making it difficult to accurately quantify the amount of bicarbonate signal lost in the lungs in each experiment.Conclusion
We have demonstrated that differences in the pyruvate-to-bicarbonate ratio between the right and left ventricles of the heart can be used to investigate alterations in gas exchange that can occur in the presence of lung injury. This method is especially useful as an additional marker of lung health for pH imaging experiments in which pyruvate and bicarbonate are already being commonly co-administered.Acknowledgements
This work was supported by the National of Institutes of Health (NIH) R01 HL124986.References
[1] N. Drachman, S. Kadlecek, M. Pourfathi, Y. Xin, H. Profka, and R. Rizi, “In vivo pH mapping of injured lungs using hyperpolarized [1-13C]pyruvate,” Magn. Reson. Med., p. n/a–n/a, Oct. 2016.
[2] Y. Lee, N. M. Zacharias, D. Piwnica-Worms, and P. K. Bhattacharya, “Chemical reaction-induced multi-molecular polarization (CRIMP),” Chem. Commun., vol. 50, no. 86, pp. 13030–13033, 2014.
[3] D. M. Wilson et al., “Multi-compound polarization by DNP allows simultaneous assessment of multiple enzymatic activities in vivo,” J. Magn. Reson., vol. 205, no. 1, pp. 141–147, Jul. 2010.
[4] D. E. Korenchan et al., “Dynamic nuclear polarization of biocompatible 13C-enriched carbonates for in vivo pH imaging,” Chem. Commun., vol. 52, no. 14, pp. 3030–3033, Feb. 2016.
[5] K. Golman and J. S. Petersson, “Metabolic imaging and other applications of hyperpolarized 13C1,” Acad. Radiol., vol. 13, no. 8, pp. 932–942, Aug. 2006.
Figures