3089
Maternal-fetal exchange and metabolism followed in real-time by dynamic hyperpolarized 13C imaging on pregnant rats1Department of Chemical Physics, Weizmann Institute of Science, Rehovot, Israel, 2NeuroSpin Centre CEA Saclay, Gif-sur-Yvette, France, 3Department of Biological Regulation, Weizmann Institute of Science, Rehovot, Israel
Synopsis
Monitoring placental metabolism is of particular interest for the early-detection of complications during pregnancy. This study discusses the use of dynamic nuclear polarization (DNP) enhanced 13C MRSI of hyperpolarized pyruvate, that has been injected into pregnant rats. The enzymatic conversion of pyruvate to lactate was followed in real-time in maternal and fetal compartments –including placentas. Lactate 13C signals in placentas could be observed; they peaked significantly later and were longer-lived than both placental 13C pyruvate and 13C signals in maternal organs. Single-voxel analyses for both metabolites in different organs revealed the T1 relaxation times and kinetics of the Pyr -> Lac transformation.
Purpose
Two thirds of all stillbirth cases are connected to placental dysfunction. Reliable, early detection of conditions such as preeclampsia could help alleviate this, but remains difficult and is currently based mainly on interpreting a combination of symptoms. The measurement of metabolic kinetics and fluxes in healthy and diseased subjects presents an opportunity to monitor the status of a pregnancy, and possibly detect these and other conditions at an early stage. In this study, dissolution dynamic nuclear polarization (dDNP)1-3 in combination with spectroscopic MRI (MRSI) is applied to pregnant rats in order to test the possibility of monitoring the nature and kinetics of maternal, placental and fetal metabolism in real-time using this emerging methodology.Methods
Seven Wistar pregnant rats were mated; at days 17 to 21 of gestation these were anesthetized with 3% of isoflurane at 1 l/min of oxygen, and a tail-vein catheter was placed for injection of hyperpolarized pyruvate. 1H MRI and 13C MRSI experiments were carried out on a Bruker Biospec 4.7T horizontal scanner. 13C-chemical-shift imaging was performed with a cross-coil configuration setup with transmission by a volume-coil and detection with a 20 mm Doty Sci surface-coil placed on the rat’s belly. 13C MRSI images were recorded using a centric chemical-shift imaging sequence4 with a TR of 68 ms, slice thickness = 1 cm, in-plane matrix sizes of 12x12 or 10x10, and a square 5 cm FOV resulting in an overall measurement time of 8.3 s or 5 s per image, respectively. These 13C images were processed using Matlab into 384 spectral data points (119 ppm bandwidth) with zero filling to 32x32 in-plane elements. 1H anatomical images were co-recorded using either surface or volume coil transmit/receive, with a gated FLASH imaging sequence targeting with a square 6 cm FOV, TE 6.3 ms and TR 615 ms. 1-13C pyruvic acid was polarized with 15mM Ox63 in an Oxford Instruments Hypersense operating at 94 GHz and 1.4K. After dissolution a 3 ml bolus of 80 mM hyperpolarized 1-13C pyruvate was injected into the rat’s tail-vein and subsequently 13C acquisitions started.Results
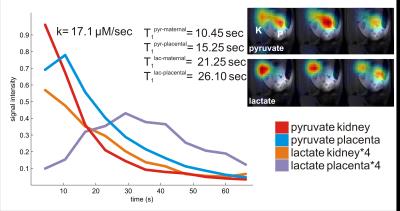
Anatomic 1H FLASH imaging enabled the identification of multiple compartments including the maternal uterine artery, vena cava and kidneys; the placentas; as well as multiple fetuses plus their livers and hearts. Series of 1-13C-pyruvate images recorded with good signal-to-noise ratio for over 60 s following injection revealed a rapid build-up and decay of the pyruvate 13C signal in the maternal compartments (uterine artery, kidney, vena cava), as well as in the placentas (Figure 1). The conversion of pyruvate to lactate could also be monitored, by the appearance of 1-13C-lactate spectral images. For the maternal kidney a rapid decay of the lactate signal is observed. By contrast a slow buildup (starting at 16 s) and eventual decay (to 56 s) is observed in the placentas (Figure 2). This is in accordance with our previous data of hyperpolarized urea, which showed slower signal decay after crossing of the placental barrier, but is at variance from observations on hyperpolarized bicarbonate which was not seen to cross at these timescales into the placenta.5 For the pyruvate signal, T1 constants were longer in the placentas than in maternal compartments; also the lactate’s apparent decay was slower in the placentas. Kinetic fits for the conversion of pyruvate to lactate in placentas resulted in an average rate constant of 17.4 µM/sec. In addition, weak, short-lived 13C-alanine signals were also observed in the fetal livers.Discussion & Conclusion
13C T1 relaxation times and kinetics of enzymatic reaction are roughly comparable to other studies that used hyperpolarized pyruvate for the monitoring of cancer.6,7 Hyperpolarized pyruvate has also been recently employed to monitor pregnancies in guinea pigs, where conversion of pyruvate to lactate has been observed in placentas and fetal livers. Pyruvate and lactate levels peaked at the same time at 20 s after injection. Also alanine was observed in maternal organs.8 Interestingly however, in our data, lactate signals could be clearly seen to peak in placentas at a later time than in maternal compartments. Thus placental disease models with a slow metabolism or different reaction kinetics might be detected in this manner. The applicability of this methodology in pregnant animals with impaired placental functionality is currently being tested.Acknowledgements
This research was supported by Minerva Project 712277, NIH grant R01HD086323, the Kimmel Institute for Magnetic Resonance and the generosity of the Perlman Family Foundation. We thank Prof. Joel Garbow (Washington Univ) for valuable discussions.References
1. Golman, K.; Zandt, R. I. T.; Thaning, M. Real-time metabolic imaging. pnas 2006, 1–6.
2. Keshari, K. R.; Wilson, D. M. Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization. Chem. Soc. Rev. 2014, 43, 1627.
3. Brindle, K. M. Imaging Metabolism with Hyperpolarized 13C-Labeled Cell Substrates. J. Am. Chem. Soc. 2015, 137, 6418–6427.
4. Zhao L, Mulkern R, Tseng CH, Williamson D, Patz S, Kraft R, Walsworth RL, Jolesz FA, Albert MS. Gradient-echo imaging considerations for hyperpolarized 129Xe MR. J Magn Reson B. 1996,113, 179-83.
5. Anne Fages, Tangi Roussel, Marina Lysenko, Ron Hadas, Michal Neeman, and Lucio Frydman. Maternal-fetal exchanges characterized by dynamic hyperpolarized 13C imaging on pregnant rats. 24th ISMRM 2016 abstract 0668.
6. Day SE, Kettunen MI, Gallagher FA, Hu DE, Lerche M, Wolber J, Golman K, Ardenkjaer-Larsen JH, Brindle KM. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med. 2007, 11, 1382-7.
7. Saito K, Matsumoto S, Takakusagi Y, Matsuo M, Morris HD, Lizak MJ, Munasinghe JP, Devasahayam N, Subramanian S, Mitchell JB, Krishna MC. 13C-MR Spectroscopic Imaging with Hyperpolarized [1-13C]pyruvate Detects Early Response to Radiotherapy in SCC Tumors and HT-29 Tumors. Clin Cancer Res. 2015, 21, 5073-81
8. Friesen-Waldner LJ, Sinclair KJ, Wade TP, Michael B, Chen AP, de Vrijer B, Regnault TR, McKenzie CA. Hyperpolarized [1-(13) C]pyruvate MRI for noninvasive examination of placental metabolism and nutrient transport: A feasibility study in pregnant guinea pigs. J Magn Reson Imaging. 2016, 43, 750-5
Figures