3088
Influence of Isoflurane Anesthesia on Assessment of Cardiac Metabolism Using Hyperpolarized [1-13C] Pyruvate1Institute for Biomedical Engineering, University and ETH Zurich, Zurich, Switzerland
Synopsis
Isoflurane is frequently used in hyperpolarized [1-13C]pyruvate studies. Even though literature suggests direct interaction with mitochondrial metabolism, the influence of the compound on cardiac metabolism has not been assessed in detail yet. In the present study the impact of low versus high isoflurane concentration is examined in a cross-over experiment. Results reveal that cardiac metabolism is modulated by isoflurane concentration showing increased lactate and reduced bicarbonate production during high isoflurane dose relative to low dose.
Introduction
Hyperpolarized [13C]pyruvate has become an
important imaging agent to investigate cardiac metabolism in experimental
animal models.1,2 For example, metabolic changes in dilated cardiomyopathy3, acute myocardial infarction2 and during treatment of diabetic cardiomyopathy4 have been demonstrated. Although
isoflurane is frequently used in experimental hyperpolarized [1-13C]pyruvate
studies and literature suggest a direct effect on mitochondrial metabolism, a
detailed examination is still lacking. The impact of isoflurane has
been examined in the context of “anesthetic preconditioning” where mitochondrial
metabolism was found to be inhibited
with a subsequent change in its redox state (increased NADH production)5–7.
The objective of the present study was to
systematically assess the influence of low versus high isoflurane concentration
on cardiac metabolism in a cross-over experiment.
Methods
Hyperpolarization
A home-built multisample dissolution dynamic nuclear polarization (DNP) system was used to polarize samples consisting of 50.8µL [1-13C]pyruvic acid and 13.5mM trityl, doped with 1mM Dotarem yielding an 80mM [1-13C]pyruvate solution after dissolution.8
Animal Preparation
All animal experiments were performed in adherence to the Swiss Animal Protection law and were approved by the regional veterinary office. The study was conducted on five healthy male Wistar rats weighing 240-396g. Rats were anesthetized with 4% isoflurane in an air-oxygen mixture (4:1) for endotracheal intubation. Thereafter ventilation was initiated and anesthesia was maintained using 1-2% isoflurane. Body temperature was kept at 37-39°C by using a water heating mat. Two 26-gauge intravenous cannulae were placed in opposite sides of the rat tail, one to allow injection of the dynamic nuclear polarization substrate and one for continuous glucose infusion (glucose, 15mg/kg/min) to keep blood glucose levels constant9.
Study Protocol
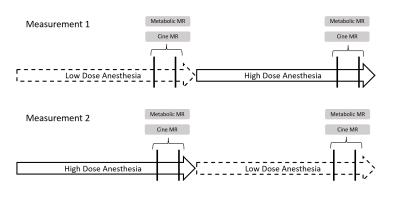
After induction, preparation and transfer into the scanner the animal was held at constant isoflurane concentration either at low dose (1.5-2%) or at high dose (3.5-4%) for approximately 1h16min±8min allowing for preparations of DNP measurements. Two successive measurements were conducted with a gap of 20min in-between. After the second measurement anesthesia was switched to the alternate isoflurane dosing for 30±5min before two further measurements were conducted, again with a gap of 20min in-between. Each animal was measured twice with alternating isoflurane dosing (Low-High and High-Low, Figure 1).
Magnetic Resonance Imaging
Imaging experiments were performed on a 9.4-T MR imaging system (Biospec 94/30, Bruker Biospin, Ettlingen, Germany). A birdcage dual 1H/13C coil (Rapid Biomedical, Wurzburg, Germany) was used for excitation. A rectangular 13C surface coil with a sensitive coil area of 40x30mm2 (Rapid Biomedical) was placed over the thorax for signal reception. Metabolic data were acquired with a multiband radiofrequency pulse in combination with a multiecho single-shot echo-planar readout10. The imaging field of view was 60x40mm2, in-plane spatial resolution was 1.25x1.25mm2, and section thickness was 4mm. Seven echoes were acquired. Metabolic imaging was triggered to end-systole, and the seven readouts were repeated every 1.5s during a total imaging duration of 2min.
Data Analysis
Metabolic images were reconstructed using the IDEAL approach11 encoding pyruvate, lactate, bicarbonate, pyruvate hydrate, and alanine resonances. Lactate, bicarbonate, and lactate-to-bicarbonate ratio were quantified based on the area under the curve (AUC) of the signal intensity–time curves. Metabolite AUCs were normalized by the total myocardial carbon (TmC) signal acquired in each scan corresponding to the sum of the lactate, bicarbonate and alanine resonances.
Results
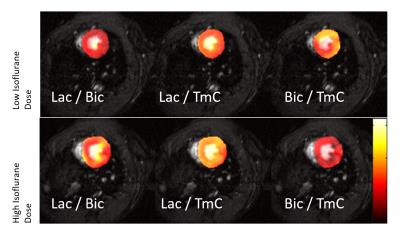
Figure 2 shows
representative images of [1-13C]lactate (Lac), [1-13C]bicarbonate (Bic) and
lactate-to-bicarbonate ratio (L2B), superimposed onto 1H-MR images.
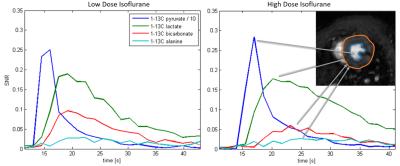
The influence of low and high dose isoflurane anesthesia is readily seen. Figure 3 displays
exemplary dynamic [1-13C]pyruvate and metabolite curves at low and high
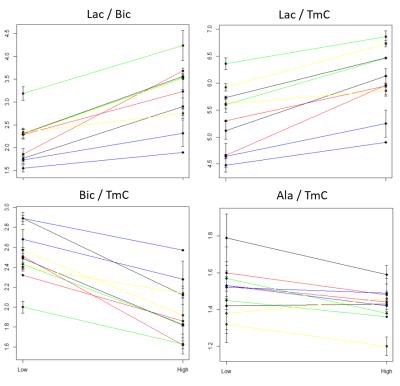
isoflurane dose. Across all animals and experiments, AUCs show an increase for Lac,
a decrease for Bic and no significant change for alanine (Figure
4). As
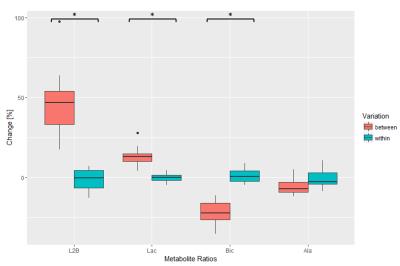
a consequence, L2B is increased. Figure 5 compares
relative changes occurring between isoflurane alternations relative to changes
in repeat measurements at constant isoflurane dose. Average changes due to
difference in isoflurane dose (L2B: 47%, Lac: 14%, Bic: -22%) differ
significantly from changes caused by method and physiological variability (L2B:
-1%, Lac: 0%, Bic: 1%). The average heart rate was 413±30bpm (ejection fraction 64±6%) for low
isoflurane dose and 367±29bpm (ejection fraction 66±7%) for the high dose.
Discussion
This present study has demonstrated that cardiac
metabolism is influenced by isoflurane concentration showing increased lactate
and reduced bicarbonate production during high isoflurane dose relative to low
dose while cardiac ejection fraction did not change significantly. This points
to the importance of reproducible anesthesia when studying modulation of
cardiac metabolism in experimental animals. Since the depth of anesthesia is
difficult to control in an experimental animal setting, careful study design is
required to exclude confounding factors.Acknowledgements
The authors acknowledge funding from the Swiss National Science Foundation, grant 320030_153014.References
1. Lau, A. Z. et al. Rapid multislice imaging of hyperpolarized 13C pyruvate and bicarbonate in the heart. Magn. Reson. Med. 64, 1323–31 (2010).
2. Oh-Ici, D. et al. Hyperpolarized Metabolic MR Imaging of Acute Myocardial Changes and Recovery after Ischemia-Reperfusion in a Small-Animal Model. Radiology 278, 742–51 (2016).
3. Schroeder, M. a. et al. Hyperpolarized 13C magnetic resonance reveals early- and late-onset changes to in vivo pyruvate metabolism in the failing heart. Eur. J. Heart Fail. 15, 130–140 (2013).
4. Le Page, L. M. et al. Increasing Pyruvate Dehydrogenase Flux as a Treatment for Diabetic Cardiomyopathy: A Combined 13C Hyperpolarized Magnetic Resonance and Echocardiography Study. Diabetes 64, 2735–43 (2015).
5. Hanley, P. J. & Loiselle, D. S. Mechanisms of force inhibition by halothane and isoflurane in intact rat cardiac muscle. J. Physiol. 506, 231–244 (1998).
6. Agarwal, B., Dash, R. K., Stowe, D. F., Bosnjak, Z. J. & Camara, A. K. S. Isoflurane modulates cardiac mitochondrial bioenergetics by selectively attenuating respiratory complexes. Biochim. Biophys. Acta - Bioenerg. 1837, 354–365 (2014).
7. Agarwal, B., Camara, A. K. S., Stowe, D. F., Bosnjak, Z. J. & Dash, R. K. Enhanced charge-independent mitochondrial free Ca2+ and attenuated ADP-induced NADH oxidation by isoflurane: Implications for cardioprotection. Biochim. Biophys. Acta - Bioenerg. 1817, 453–465 (2012).
8. Krajewski, M. et al. A multisample dissolution dynamic nuclear polarization system for serial injections in small animals. Magn. Reson. Med. (2016). doi:10.1002/mrm.26147
9. Lauritzen, M. H. et al. Enhancing the [13C]bicarbonate signal in cardiac hyperpolarized [1-13C]pyruvate MRS studies by infusion of glucose, insulin and potassium. NMR Biomed. 26, 1496–1500 (2013).
10. Sigfridsson, A. et al. Hybrid multiband excitation multiecho acquisition for hyperpolarized 13 C spectroscopic imaging. Magn. Reson. Med. 73, 1713–1717 (2015).
11. Reeder, S. B. et al. Multicoil Dixon chemical species separation with an iterative least-squares estimation method. Magn. Reson. Med. 51, 35–45 (2004).
Figures