3086
Assessment of metabolism in early renal ischemia/reperfusion injury using hyperpolarized 13C-pyruvate.1MR Research Center, Aarhus University Hospital, Aarhus N, Denmark
Synopsis
Renal IRI is a leading cause of AKI in several disease states; there is a current lack of precise methods to directly assess success of kidney transplant after reperfusion. We here showed how we can measure metabolic function in both the contralateral kidney and post-ischemic 2 min after reperfusion and again after 1 hour of reperfusion. We here saw a very different response compared to metabolic data collected from animals after 24 hours of reperfusion. In this study we induced mild/moderate ischema and it looks like we captured metabolic images in a phase of repair and salvage to maintain normal kidney filtration function in the animals. It seems that severe injury has not yet occurred, or maybe won’t occur. Together with perfusion measurements or kidney filtration measurements this method might hold some clinical value.
Purpose
Renal ischemia/reperfusion injury (IRI) makes up 47% of all cases of acute kidney injury (AKI)1, and up to 1.9% of all hospitalized patients develop AKI2. The illness is especially common in critically ill patients, and the prevalence in this group is > 40% at admission to the intensive-care unit if sepsis is present2. AKI is typically seen in conjunction with other disease states, including hypovolemic shock, sepsis and surgery. Imbalance in energy metabolism and mitochondrial function is a hallmark in IRI which can be caused by mechanisms like oxidative stress, apoptosis and inflammation1. We have recently published a paper showing how metabolic profile change in severe unilateral IRI, but does not change significantly in mild/moderate IRI, although kidney function is reduced3. In this study we wanted to investigate the metabolic profile immediately after reperfusion and after an hour of reperfusion, to dissect metabolic alterations after ischemia and after reperfusion.Methods
Six Wistar Rats were included in the study. Rats were subjected to unilateral renal ischemia in the scanner for 30 min followed by reperfusion for 2 min where 13C-pyruvate was injected. 13C-pyruvate was again injected after 1 hour of reperfusion. A tail vein catheter was inserted for injection of hyperpolarized [1-13C]pyruvate. A midline incision in the abdomen was made and the left renal artery was carefully dissected. An inflatable clamp was placed around the renal pelvis, and secured with ligature on the underlining muscle layer. After the animal was placed in the scanner the clamp was inflated, and pressure was released after 30 min giving rise to 30 min of ischemia. Temperature, arterial oxygen saturation and respiration rate were monitored throughout the experiment. Each animal received one injection of 1.1 mL hyperpolarized [1-13C]pyruvate over 10 s. The experiments were performed in a 3 T clinical MR system (GE Healthcare) equipped with a dual tuned 13C/1H volume rat coil. A slice-selective 13C IDEAL spiral sequence was used for hyperpolarized [1-13C]pyruvate imaging acquiring images every 5 s initiated 20 s after the start of injection. Flip angle=10º, 11 IDEAL echoes and one initial spectrum per IDEAL encoding, TR/TE/ΔTE=100 ms/0.9 ms/0.9 ms, FOV=80x80 mm2, 5 x 5 mm real resolution and an axial slice thickness of 15 mm covering both kidneys.Results and discussion
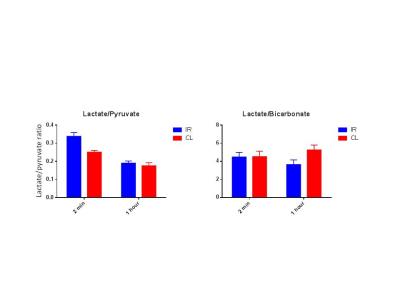
We found a statistical significant difference between kidneys and in time in the lactate/pyruvate ratio. Albeit did not see any difference in interaction. We interpret this as an initial repair phase mechanism where metabolism is elevated in both kidneys because of the mild/moderate ischemia. We find a statistical significant difference in the interaction of the lactate/bicarbonate ratios. After 24 hours of reperfusion we have previously seen an elevation in anaerobic metabolism if severe injury has been induced, or no difference in anaerobic metabolism compared to the contralateral if mild/moderate injury has been induced3. Here we find a completely new situation where there is a reduction in anaerobic metabolism in the post-ischemic kidney and an elevation in the contralateral kidney. This probably means that kidneys have not received severe injury either because of the short ischemic period or the short reperfusion period, or even both. This is very interesting and should be further investigated by measuring relevant enzyme activities, mRNA transcription levels and relevant injury markers.Conclusion
In conclusion we here found an initial elevation lactate/pyruvate ratio in both kidneys after 30 min of ischemia and 2 min of reperfusion. This probably denotes a chock period after the ischemia which is seen in both kidneys. After 1 hours of reperfusion we see a reduction in anaerobic metabolism in the post-ischemic kidney, and an elevation in the contralateral kidney. This is probably an early repair response in the kidneys, to keep total animal kidney filtration normal.Acknowledgements
Henrik Vestergaard is acknowledged for his laboratory assistance.References
1. Hoste, E. A. J. et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 41, 1411–1423 (2015).
2. Bellomo, R., Kellum, J. A. & Ronco, C. Acute kidney injury. Lancet 380, 756–766 (2012).
3. Nielsen P.M. et. al. In situ lactate dehydrogenase activity - a novel renal cortical imaging biomarker of tubular injury? Am J Physiol Renal Physiol [Ahead of print](2016).