3085
Measuring perfusion in a renal ischemic/reperfusion rat model using hyperpolarized α-Trideuteromethyl[15N]glutamine.1MR Research Center, Aarhus University Hospital, Aarhus N, Denmark, 2GE Global Research, Munich, Germany
Synopsis
Renal IRI is a leading cause of AKI in several disease states; currently there are several methods to measure renal perfusion in the clinic, but all suffer under specific drawbacks. Here we present a pilot study using the hyperpolarized perfusion marker α-trideuteromethyl[15N]glutamine in a 40 min unilateral ischemia reperfusion rat model. A reduction of 51% in perfusion was observed in the animal. We therefore believe that α-trideuteromethyl[15N]glutamine is a highly promising molecule in renal perfusion studies.
Purpose
Renal ischemia/reperfusion injury (IRI) makes up 47% of all cases of acute kidney injury (AKI)1, and up to 1.9% of all hospitalized patients develop AKI2. The illness is especially common in critically ill patients, and the prevalence in this group is > 40% at admission to the intensive-care unit if sepsis is present2. AKI is typically seen in conjunction with other disease states, including hypovolemic shock, sepsis and surgery. Imbalance in energy metabolism and mitochondrial function is a hallmark in I/R-I which can be caused by mechanisms like oxidative stress, apoptosis and inflammation1. Evaluation of renal perfusion is vital in these disease cases especially after trauma and kidney transplantation. There is currently an array of noninvasive tests to measure renal perfusion in a clinical setting, but these suffer under several drawbacks like contrast-induced nephropathy and ionizing radiation. [13C,15N2]-Urea have been proposed as a hyperpolarized perfusion marker in animal studies3, albeit suffer under a rapid signal decay caused by the short T1 relaxation time. In this pilot study we utilized the newly developed α-Trideuteromethyl[15N]glutamine4 which have a particular long T1 relaxation time to measure perfusion in a renal unilateral ischemic/reperfusion injury (IRI) rat model.Methods
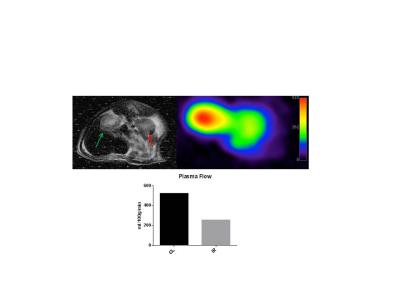
Four rats underwent unilateral renal ischemia for 40 min followed by reperfusion for 24 hours. All rats were put in metabolic cages before and after surgery. One of these rats were injected with hyperpolarized α-Trideuteromethyl[15N]glutamine, the contralateral healthy kidney served as control. A midline incision in the abdomen was made and the left renal artery was carefully dissected. IRI was induced by clamping the left artery with a non-traumatic clamp for 40 min. Temperature and respiration was monitored during the surgical procedure. Data from metabolic cages were collected 24 hours before surgery and 24 hours after surgery. A tail vein catheter was inserted for injection of hyperpolarized α-Trideuteromethyl[15N]glutamine before the MRI scanning. Each animal received one injection of 1.1 mL hyperpolarized α-Trideuteromethyl[15N]glutamine over 10 s. The experiments were performed in a 3 T clinical MR system (GE Healthcare) equipped with 1H head coil and a 15N rat coil. A slice-selective 15N spiral sequence was used for hyperpolarized α-Trideuteromethyl[15N]glutamine imaging acquiring images every 1 second initiated at the start of injection for 180 seconds. Flip angle=15º, FOV=80x80 mm2, 5 x 5 mm real resolution and an axial slice thickness of 20 mm covering both kidneys.Results and discussion
IRI resulted in decreased water and food intake, also urine output and kidney weight increased. Plasma flow was reduced by 268ml/100g/min compared to the contralateral kidney (CL). All animals showed signs of AKI as they have reduced food and water intake, albeit still had an elevated urine output. This is probably caused by a reduced osmolality gradient leading to a reduced ability to concentrate urine. The apparent reduced perfusion agrees with studies performed by Zimmer et. al.5 Additional animals will be included in the study to assess the usefulness of α-trideuteromethyl[15N]glutamine as a new perfusion tracer in AKI. However the preliminary data indicate, that hyperpolarized α-trideuteromethyl[15N]glutamine is a promising marker of renal perfusion in AKI. We speculate that as α-trideuteromethyl[15N]glutamine is an artificial molecule it will probably be filtered freely in the glomeruli with no secondary uptake giving rise to a possible glomerular filtration rate (GFR) capabilities, however further studies are needed to confirm the ability to accurately determine GFRConclusion
In conclusion, we were able to detect a difference in perfusion between the post-ischemic kidney and the contralateral kidney using the newly developed α-trideuteromethyl[15N]glutamine molecule which provides a significantly higher T1 relaxation time compared to [13C,15N2]-Urea.Acknowledgements
Henrik Vestergaard is acknowledged for his laboratory assistance.References
1. Hoste, E. A. J. et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 41, 1411–1423 (2015).
2. Bellomo, R., Kellum, J. A. & Ronco, C. Acute kidney injury. Lancet 380, 756–766 (2012).
3. Nielsen, P. M. et al. Renal ischemia and reperfusion assessment with three-dimensional hyperpolarized (13) C,(15) N2-urea. Magn. Reson. Med. [Ahead of print] (2016).
4. Durst, M. et al. α-trideuteromethyl[15N]glutamine: A long-lived hyperpolarized perfusion marker. Magn. Reson. Med. [Ahead of print] (2016).
5. Zimmer, F. et al. Quantitative Renal Perfusion Measurements in a Rat Model of Acute Kidney Injury at 3T: Testing Inter- and Intramethodical Significance of ASL and DCE-MRI. PLoS One 8, (2013).
Figures