3083
Assessment of lactate dehydrogenase activity in renal cell carcinomas using hyperpolarized 13C pyruvate MR1Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States
Synopsis
The incidence of renal cell carcinomas (RCCs) has increased significantly over time due to the widespread use of cross-sectional imaging with incidental cancer detection. RCCs vary widely in histological grade and risk of metastasis. However, current imaging techniques cannot reliably differentiate low grade, indolent RCCs from localized but potentially aggressive RCCs, resulting in the over-treatment of many indolent cancers. Increasing evidence has shown that increased glycolysis with lactate production is a dominant metabolic feature of RCCs. In particular, lactate dehydrogenase expression is positively correlated with RCC grade, and a strong predictor of tumor progression and poor prognosis. Hyperpolarized 13C MR allows real time investigation of cellular metabolism, and provides time-resolved metabolic kinetics that reflects flux through enzyme-catalyzed reactions. The purpose of this study is to investigate whether hypeprolarized 13C pyruvate MR can inform on the LDH activity in orthotopic RCC tumor models.
Purpose
The incidence of renal cell carcinomas (RCCs) has increased significantly over time due to the widespread use of cross-sectional imaging with incidental cancer detection. RCCs vary widely in histological grade and risk of metastasis. However, current imaging techniques cannot reliably differentiate low grade, indolent RCCs from localized but potentially aggressive RCCs, resulting in the over-treatment of many indolent cancers. Increasing evidence has shown that increased glycolysis with lactate production is a dominant metabolic feature of RCCs. In particular, lactate dehydrogenase expression is positively correlated with RCC grade, and a strong predictor of tumor progression and poor prognosis. Hyperpolarized (HP) 13C magnetic resonance (MR) allows real time investigation of cellular metabolism, and provides time-resolved metabolic kinetics that reflects flux through enzyme-catalyzed reactions. The purpose of this study is to investigate whether HP 13C pyruvate MR can inform on lactate dehydrogenase (LDH) activity in orthotopic RCC tumor models.Methods
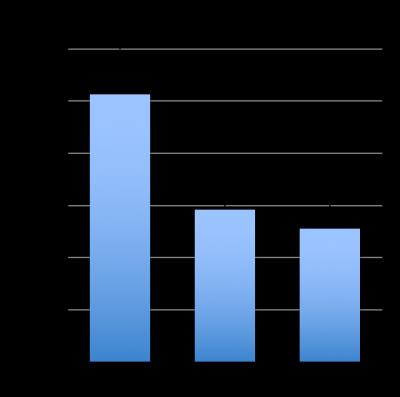
Three RCC cell lines with varying LDHA expression were chosen to create the orthotopic tumors in mice. Figure.1 shows the RNA expression levels determined using RT-qPCR of cells grown in monolayers in tissue culture flasks. A498 (n=2) and 786-O (n=2) were both derived from clear cell RCCs, and UOK262 (n=3) was derived from a type 2 papillary RCC. Five to ten million cells were injected under the renal capsule of immune deficient male mice (4-6 weeks old). Tumors were detected in 6-8 weeks post implantation and were imaged using HP 13C pyruvate MR when they reached a volume of at least 0.2 cc. 350µl of 80mM of hyperpolarized [1-13C]pyruvate was injected via a tail vein catheter over 10-12 sec. Dynamic, spectrally and spatially selective RF pulses were used to acquire hyperpolarized 13C pyruvate and lactate images on a 14T vertical bore Agilent spectrometer equipped with a dual tune (M2M, Australia) 40mm coil, with a repetition time of 3s and a total of 20 images. Dynamic 13C lactate images were acquired using a 90° pulse, while 13C pyruvate images were acquired using a variable flip angle (from 3-90°). Data were corrected for the RF flip angle and processed using Matlab (Mathworks, Boston, USA), and represented as area under the curve of 13C lactate to maximal pyruvate signal (AUC lac/max pyr). T2 weighted and diffusion weighted proton images were also acquired for anatomic reference and proton ADC map generation, respectively. At the end of the imaging, the animals were euthanized and the tumor tissue was quickly harvested for immunohistochemical (IHC) staining and biochemical assays. Tissue staining, including H&E, Ki67 and Hu70 for morphology, proliferation and human cell identification was performed on all tumors. LDHA expression as well as LDH activity from the harvested tumor tissue were also measured.Results
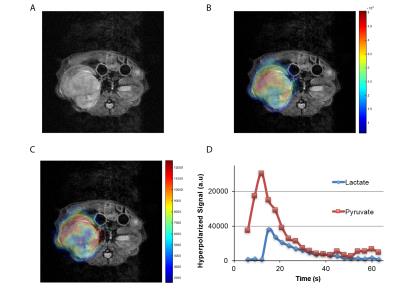
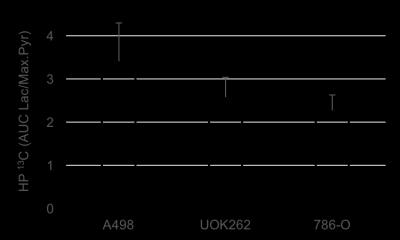
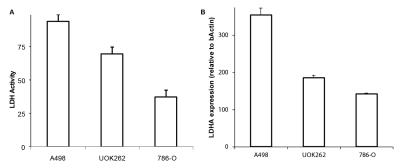
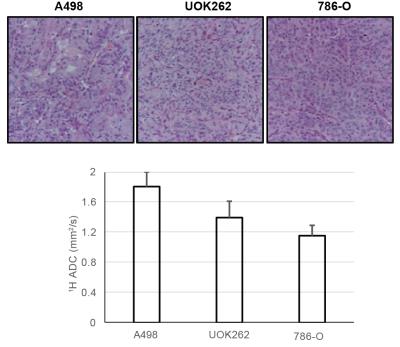
Figure.2 shows a representative T2-weighted image of an orthotopic RCC tumor (UOK262), with overlaid 13C lactate (15s post infusion) and 13C pyruvate (maximal signal at ~12s) images. The graph shows the corresponding dynamic lactate and pyruvate signal in the tumor. Figure 3 shows the mean ratio of HP 13C AUC lac/max pyr, normalized to the tumor proton ADC value of the 3 RCC tumors. The A498 tumors showed the highest lactate signal, followed by UOK262 tumors, and lastly the 786O tumors, though small sample size precludes statistical testing. Figure 4 shows the LDH activity and expression of the harvested tumor tissue, with trends that correspond to the LDH expression level in cell culture. The HP 13C AUC lac/max pyr ratios of the tumors in vivo correlate with the LDH activity and expression measured from the tumor tissue. Of note, the hyperpolarized lactate data were normalized to the proton ADC values of the tumors to account for the different tumor cell density in vivo. The varying tumor cell density is evident from the IHC stain (fig.5) which is inversely related to measured mean 1H ADC of the in vivo tumors as seen in RCC clinically1.Discussion and Conclusion
Our initial data suggest that HP 13C pyruvate to lactate conversion can inform on the LDH enzyme activity in RCC tumors. Studies are ongoing to confirm the finding in additional mice. As LDH has been shown to correlate with RCC grade and patient prognosis, this technique may provide a noninvasive means to differentiate indolent from potentially aggressive RCCs.Acknowledgements
We would like to acknowledge Sukumar Subramaniam, Romelyn DeLos Santos, Jessie Lee, Jinny Sun and Dave Korenchan for their help with experiments.
Grants: NIH P41EB013598 (JK), Department of Defense Peer Reviewed Visionary Postdoctoral Fellowship (RS), Department of Defense Peer Reviewed Cancer Research Concept Award (ZJW), Radiological Society of North America Scholar grant (ZJW).
References
1. Malignant renal neoplasms: correlation between ADC values and cellularity in diffusion weighted magnetic resonance imaging at 3 T.Manenti G, Di Roma M, Mancino S, Bartolucci DA, Palmieri G, Mastrangeli R, Miano R, Squillaci E, Simonetti G.Radiol Med. 2008 Mar;113(2):199-213. doi: 10.1007/s11547-008-0246-9Figures