3082
Study of the Tetracycline-controlled Transcriptional Activation of c-Myc in Burkitt Lymphoma B-cell Line P493-6 Using Hyperpolarized [1-13C]pyruvate1Department of Nuclear Medicine, Klinikum rechts der Isar, Technische Universität München, Munich, Germany, 2Department of Chemistry, Technische Universität München, Munich, Germany, 3GE Global Research, Munich, Germany, 4III. Medical Department, Klinikum rechts der Isar, Technische Universität München, Munich, Germany
Synopsis
We proved that the transcriptional activation of c-Myc expression controlled by tetracycline has a direct influence on lactate dehydrogenase-A (LDH-A) activity in B-cell line P493-6 in vitro. Using hyperpolarized [1-13C]pyruvate and 13C magnetic resonance spectroscopy we were able to monitor a reduction of pyruvate to lactate reaction catalyzed by LDH-A. Incubation of the P493-6 cells in a media with 0.1 μg/mL tetracycline for 24 hours reduced the kinetic value of the reaction by 41.8±10.5 %. This proves that the control of c-myc has a significant influence on LDH-A activity and can be measured using 13C magnetic resonance spectroscopy with hyperpolarized [1-13C]pyruvate.
Purpose
The aim was to study the influence of tetracycline-controlled transcriptional activation of c-Myc on lactate dehydrogenase-A (LDH-A) expression in Burkitt lymphoma B-cell line P493-6 using hyperpolarized [1-13C]pyruvate (PA) and 13C magnetic resonance spectroscopy (13CMRS).Introduction
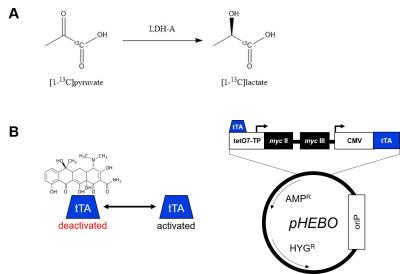
Approximately 70,000 cancer deaths per year are associated with mutations in the c-Myc gene in the USA.1 c-Myc plays a crucial role in the regulation of cell growth control, proliferation, differentiation, and apoptosis.2,3 Shim et al. (1997) proved that c-Myc is responsible for a direct increase of LDH-A expression. LDH-A is an enzyme catalyzing a reaction of pyruvate to lactate under anaerobic conditions (Fig.1A). Elevation of LDH-A expression is thought to be one of the main indication of cancer cells, which unlike healthy cells produce lactate anaerobically, a phenomenon known as the Warburg effect. Its overexpression alone is sufficient to increase pyruvate to lactate turnover.4 Burkitt lymphoma B-cell line P493-6 carries conditional myc gene allowing myc expression regulation using a tetracycline-regulated promoter (Fig.1B). Re-expression of myc activates the cell cycle without inducing apoptosis.5 Dynamic nuclear polarization of 13C-labeled compounds enhances the NMR signal of 13C by over 10,000-fold.6 In combination with 13CMRS, this method allows studying different steps of metabolic pathways in vitro as well as in vivo. Over past decade, it has proven to be a very successful method to study the pyruvate to lactate turnover as well as other metabolic reactions.7Methods
A mixture of [1-13C]pyruvic acid (CIL, Massachusetts, USA), 15 mmol/L trityl radical OX063 (GE Healthcare, Amersham, UK), and 1.4 mmol/L Dotarem® (Guerbet, Roissy, France) was hyperpolarized using Hypersense™ polarizer (Oxford Instruments, Abingdon, UK) at ~94 GHz. The sample was dissolved in PBS buffer to 20 mmol/L at pH 7.4. The solution was mixed with the P493-6 cell suspension (cell number = 4×107, 6×107, 8×107, 108) resulting in 5 mmol/L final [1-13C]pyruvate concentration. The mixture was measured at 1 T Spinsolve® NMR spectrometer (Magritek, San Diego, USA) using FA = 10°, TR = 3s, NS = 100. P493-6 cells were cultured in RPMI 1640 medium supplemented with fetal calf serum (10%), L-glutamine (2 mmol/L), penicillin (100 units/mL), and streptomycin (100 μg/mL). For the treatment/repression of myc, tetracycline (0.1 μg/mL) was added to culture medium and after 24 hours a measurement was performed.5 The kinetic reaction was modeled using a fitting model based on Harrison et al. (2012).8Results & Discussion
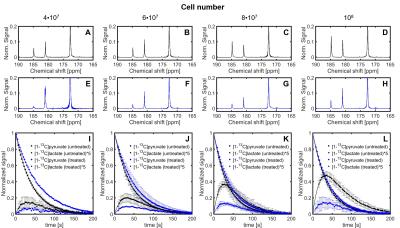
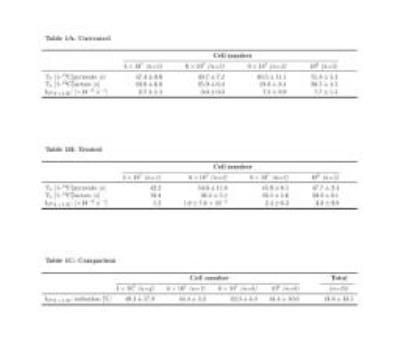
Sequences of 13C spectra were obtained for mixtures of hyperpolarized [1-13C]pyruvate (PA) with different cell amounts. Figure 2 shows a sum of spectra between time 15 and 35 s after the mixing of hyperpolarized [1-13C]pyruvate with different amounts of untreated P439-6 cells (Fig.2A-D) and cells after 24 hours treatment with 0.1 μg/mL tetracycline (Fig.2E-H). The decay of the signal intensity, as well as the kinetic model, fitting is displayed in the Figure 2I-L. The spin–lattice relaxation times (T1) obtained during the in vitro measurements were 50.1 ± 7.1 s for PA (n=25) and 25.9 ± 5.9 s (n=25) for [1-13C]lactate (LAC) at 1 T. There was no significant difference between the T1´s of untreated cells T1(PA) = 50.0±6.5 s, T1(LAC) = 25.8±5.9 s (n=16) and treated cells T1(PA) = 50.3±8.2 s, T1(LAC) = 26.1±6.2 s (n=9). Using a non-linear kinetic modeling, we calculated a kinetic value of the pyruvate to lactate reaction (kPA→LAC), and the results are reported in Table 1A-B for both treated and untreated cells. There is an increasing trend of kPA→LAC with increasing cell number in both untreated and treated cells (Fig.3A) indicating that the uptake and the pyruvate to lactate reaction is sufficiently supplied by [1-13C]pyruvate and that its concentration (4 mmol/L) is above the maximum amount able to be processed by the cell amounts examined. The 24 hours’ incubation of the cells with 0.1 μg/mL tetracycline shows a significant decrease of kPA→LAC by 41.8±10.5 % (n=25) (Fig.3C). The kPA→LAC reduction for each cell amount is reported in Table 1C as well as in Figure 3B.Conclusion
We measured a reduction of the metabolic reaction of pyruvate to lactate by 41.8±10.5 % caused by incubation of the P493-6 cells in the media with 0.1 μg/mL tetracycline. This proves that the control of the c-myc expression using tetracycline has a significant influence on LDH-A expression and that 13C magnetic resonance spectroscopy with hyperpolarized [1-13C]pyruvate is a method that is able to measure this phenomenon. We continue with these measurements in vivo using a mouse model.Acknowledgements
Funded by SFB824 Teilprojekt A5 and C3References
1. Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol. 1999;19(1):1-11.
2. Henriksson M, Lüscher B. Proteins of the Myc network: essential regulators of cell growth and differentiation. Adv Cancer Res. 1996;68:109-182.
3. Schuhmacher M, Kohlhuber F, Hülzel M, et al. The transcriptional program of a human B cell line in response to Myc. Nucleic Acids Res 2001;29(2):397-406.
4. Shim H, Dolde C, Lewis BC et al. c-Myc transactivation of LDH-A: Implications for tumor metabolism and growth. Proc Natl Acad Sci U.S.A. 1997;94(13):6658-6663.
5. Pajic A, Spitkovsky D, Christoph B, et al. Cell cycle activation by c-myc in a Burkitt lymphoma model cell line. Int J Cancer 2000;87(6):787-793.
6. Ardenkjær-Larsen JH, Fridlund B, Gram A, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U.S.A. 2003;100(18):10158-10196.
7. Keshari KR, Wilson DM. Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization. Chem Soc. Rev. 2014;7;43(5):1627-1659.
8. Harrison C, Yang C, Jindal A et al. Comparison of kinetic models for analysis of pyruvate-to-lactate exchange by hyperpolarized 13C NMR. NMR Biomed. 2012;25:1286-1294.
Figures