3081
Evaluation of the in vivo on-target effect of a newly developed LDH inhibitor using hyperpolarized 13C Magnetic Resonance Spectroscopic Imaging1RBB,UOB/NCI, NIH, Bethesda, MD, United States, 2RBB/NCI, NIH, Bethesda, MD, United States, 3UOB/NCI, NIH, Bethesda, MD, United States, 4National Center for Advancing Translational Sciences (NCATS), NIH, Rockville, MD, United States
Synopsis
This study aimed to monitor the impact on metabolic flux in vivo of a newly developed Lactate Dehydrogenase A Inhibitor (LDHI), using hyperpolarized 13C Magnetic Resonance (MR) technology. Using hyperpolarized 13C MR Spectroscopy and Chemical Shift imaging, we found that the LDHI significantly and rapidly suppressed the [1-13C]lactate to [1-13C]pyruvate ratio after single dose administration to mice harboring a MiaPaca (a glycolytic pancreatic cancer cell line) xenograft. These results indicate that the LDHI suppressed lactate production in the tumors. Thus, using Hyperpolarized 13C MRI provides a very useful technology to evaluate in vivo on-target efficacy of LDH inhibitors.
Purpose
Increased lactate production is a characteristic metabolic perturbation in many types of cancer and is known as the “Warburg effect”(1, 2). Accelerated lactate production is a consequence of increased conversion from pyruvate by Lactate Dehydrogenase A (LDHA). Based on genetic studies, LDHA inhibition is considered to be a promising strategy to develop a new therapeutic approach to treat cancers bearing the Warburg phenotype. For this purpose, one requirement is a drug that can inhibit LDHA in vivo, while a feasible and sensitive non-invasive imaging approach that can dynamically evaluate the drug’s in vivo on-target efficacy would also be highly beneficial.
Hyperpolarized 13C Magnetic Resonance Imaging (MRI) is a valuable technology to investigate metabolic processes in tumor xenografts, allowing us to perform dynamic 13C-metabolic flux analysis in vivo. Use of [1-13C]pyruvate with this technology provides the ability to monitor LDHA activity through dynamic observation of conversion of [1-13C]pyruvate to [1-13C]lactate.
Herein, we demonstrate application of this technique to quantify in vivo on-target efficacy of a newly developed LDH inhibitor (LDHI) in a glycolytic xenograft tumor model.
Methods
LDH inhibitor: The LDH inhibitor was obtained from NCATS by agreement with National Cancer Institute (NCI) Experimental Therapeutics Program (NExT).
Native Gel assay: Native gel assay was performed as described previously(3). Samples were subjected to 6% Tris-glycine gel electrophoresis. After electrophoresis, the gel was soaked in LDHI for 30 min and then incubated with reaction buffer at 42C for 7-14 minutes.
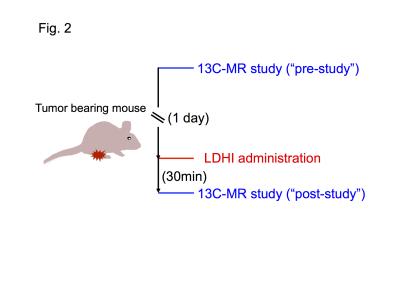
Hyperpolarized 13C-MRI study: Hyperpolarized 13C-MRI study was performed as described previously(4). [1-13C]pyruvate (30 μL), containing 15 mmol/L OXO63 and 2.5 mmol/L gadolinium chelate, was hyperpolarized using the Hypersense DNP polarizer. The hyperpolarized sample was rapidly dissolved in 4.5 mL of a superheated alkaline buffer, and dissolved solution was intravenously injected (12 μL/g body weight). Hyperpolarized 13C MRI studies were performed on a 3T scanner using a 17 mm home-built 13C solenoid coil placed inside of a saddle coil for 1H. The 13C MR spectra were acquired every second with a spectral width of 3330 Hz, repetition time of 1000ms and flip angle of 10°. 13C two-dimensional spectroscopic images were acquired 27 seconds after the start of the pyruvate injection, with a 28 × 28 mm2 field of view in a 8 mm axial slice, a matrix size of 14 × 14. Initial 13C MRI evaluation was performed in a mouse harboring a MiaPaca tumor, 1 day before LDHI injection (“pre-study”), and again 30 min after LDHI injection (“post-study”). The LDHI was intravenously injected (50 mg/kg).
Results
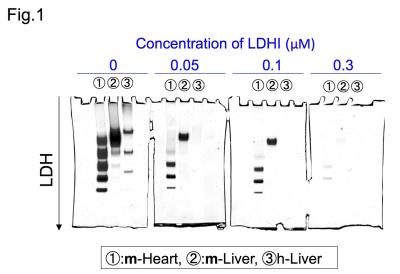
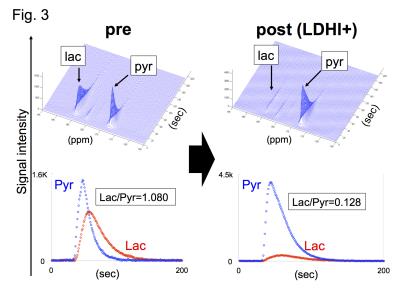
Native gel assay showed that LDHI dose-dependently suppressed in-gel redox activity of human LDH isoforms (Fig. 1). Similar results were obtained for LDH proteins in mouse tissues. Hyperpolarized MR study with [1-13C]pyruvate was performed before and after LDHI administration to assess inhibitor impact on metabolic flux in vivo. A schematic representation of hyperpolarized [1-13C]pyruvate MR study is seen in figure 2. 13C MR spectroscopy revealed that lactate production in the tumor was obviously suppressed 30 minutes after intravenous administration of the LDHI, and that [1-13C]lactate to [1-13C]pyruvate ratio ([1-13C]-Lac/Pyr), which was calculated from the areas under the curves (AUC) in the time-intensity curves, decreased from 1.08 to 0.128 (88.1% decrease) (Fig. 3). In addition, Chemical Shift Imaging with 13C MRI showed that [1-13C]lactate signal in each voxel was remarkably decreased in the tumor region upon LDHI administration (Fig. 4). Furthermore, the sum of [1-13C]lactate signals in the tumor region significantly decreased after the LDHI administration (Fig. 5A), and [1-13C] Lac/Pyr ratio calculated from chemical shift images in total tumor region significantly decreased from 1.463±0.31 to 0.134±0.036 (90.67±2.56% decrease, n=3, p<0.01) (Fig. 5B).
Conclusions
In the current study, intratumoral inhibition of LDHA in vivo upon intravenous administration of LDHI was readily visualized as markedly reduced conversion of [1-13C]pyruvate to [1-13C]lactate. These findings confirm that hyperpolarized 13C-MRI is a useful technology to evaluate on-target in vivo efficacy of LDHI. The current method can be of great value in providing a non-invasive in vivo pharmacodynamic assessment of agents such as LDHI that target deregulated tumor metabolism.Acknowledgements
This project was supported in part with funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E, through the NExT Chemical Biology Consortium
References
(1) Warburg O, et al. Ueber den Stoffwechsel der Tumoren. Biochemische Z. 1924, 152:319-344
(2) Warburg O. On the origin of cancer cells. Science, 1956, 123(3191):309-14.
(3) Ilka W, et al. Native electrophoretic techniques to identify protein-protein interactions. Proteomics, 2009, 9, 5214–23
(4) Saito K, et al. 13C-MR Spectroscopic Imaging with Hyperpolarized [1-13C]pyruvate Detects Early Response to Radiotherapy in SCC Tumors and HT-29 Tumors. Clin Cancer Res, 2015, 21(22):5073-81
Figures
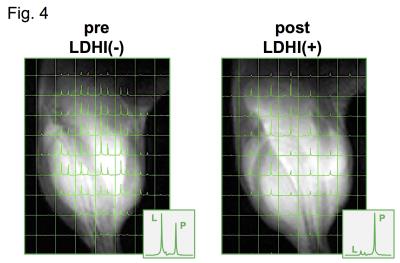
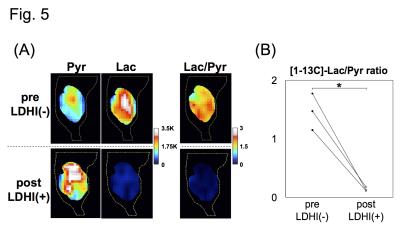
(A) Images of [1-13C]pyruvate(left), [1-13C]lactate (middle) and the [1-13C]lactate to [1-13C]pyruvate ratio (right, [1-13C]Lac/Pyr), in pre- (upper) and post- (lower) evaluations. (B) [1-13C]Lac/Pyr calculated from the chemical shift images in pre- and post- evaluations.