3078
Detection of metastasis-associated macrophages in the lung, lymph nodes and brain using fluorine-19 based MRI cell tracking1Medical Biophysics, Western University, London, ON, Canada, 2Robarts Research Institute, London, ON, Canada
Synopsis
The escape and invasion of cancer cells and the growth of metastatic tumors is in part due to the role of tumor associated macrophages. The macrophages present in these metastatic sites are called metastasis associated macrophages. This study used 19F-based cellular MRI to detect lymph node, lung and brain metastases arising from breast cancer. A custom built 1H/19F birdcage coil allowed for ‘head to toe’ mouse imaging, allowing for detection of 19F agent accumulation, with 1H images verifying anatomical location. This information may be useful in understanding the timing and role of macrophages in the metastatic process.
Introduction
Accumulating evidence shows that tumor associated macrophages (TAMs) have a pivotal role in breast cancer growth and metastasis. The cellular composition of some breast tumors can be up to 50% TAMs.1 Metastases from breast cancer occur in the lung, liver, lymph nodes, bone and brain. Several studies have shown that macrophages are recruited to distant sites to support the growth of metastases, participating in the “premetastatic niche”. Recently Kitamura et al. demonstrated macrophage retention in breast cancer lung metastases.2 Macrophages within metastases have a distinct phenotype and are known as metastasis-associated macrophages (MAMs). In this paper we present the first evidence that 19F-based MRI cell tracking can detect MAMs in breast cancer metastases that develop within the lung, lymph nodes and brain.Methods
4T1 murine breast cancer cells (300,000) were injected orthotopically into the mammary fat pad in female BALB/c mice. 4T1 tumors generate spontaneous metastases in the lungs and lymph nodes. A brain-seeking version of the 4T1 cell line (4T1BR5) was used to generate brain metastases by injection of 20,000 cells via intracardiac injection. 1H and 19F images were acquired on a 9.4T small animal MRI scanner. A dual-tuned 1H/19F birdcage coil was constructed to allow head to toe mouse imaging, essential to visualize metastases in the whole body. Images were acquired 24 hours post IV injection of a red fluorescent PFC agent at 4 weeks post cell injection for 4T1, and 2 weeks post cell injection for 4T1BR5. Spatial resolution was 0.5x0.5x1.0 mm3 (19F) and 200x200x200 micron3 (1H). Mice were euthanized immediately following MRI and lungs, brains and lymph nodes showing 19F signal were excised. Tissues were examined using fluorescence microscopy to detect the red fluorescence of the PFC and green F4/80-FITC antibody to identify macrophages.Results
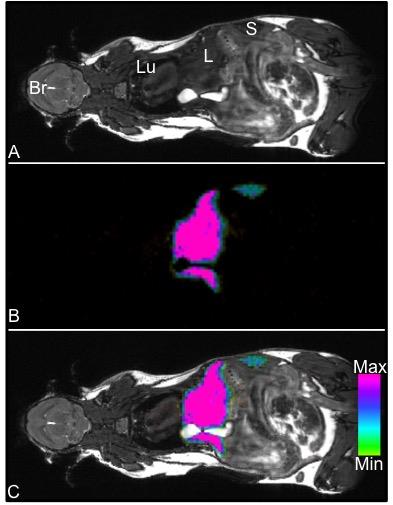
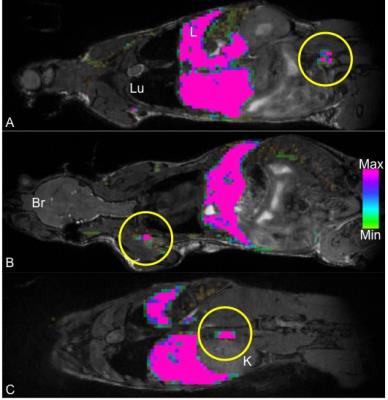
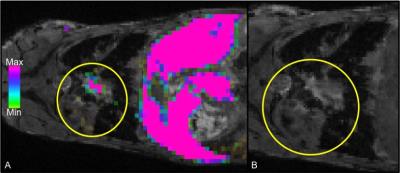
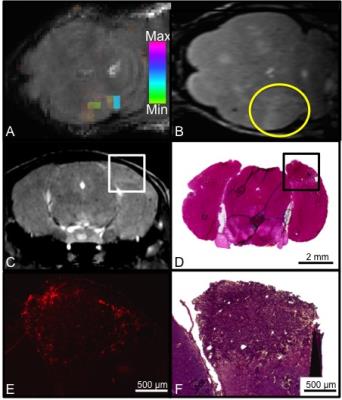
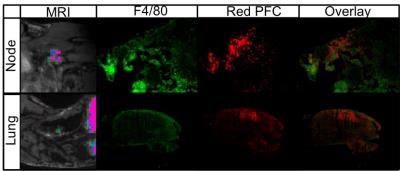
Figure 1 demonstrates whole body 1H (1A) and 19F (1B) MRI images acquired with a 9.4T MRI system. An overlay (1C) can then be created for anatomical reference of the 19F signal detected within the mouse body. In this figure, 19F signal is observed in the liver and spleen due to uptake of the PFC agent by resident macrophages. No background 19F signal is observed in other tissues. In mice with primary 4T1 tumors we observed 19F signal in lymph nodes and the lungs. Figure 2 shows images of 19F signal in ipsilateral axillary, renal and lumbar lymph nodes. These nodes were seen to be enlarged in 1H images. 19F signal was not detected within lymph nodes on the contralateral side of the mouse. Figure 3 displays 19F signal detected in lung metastases (3A - outlined in yellow). 1H images show hyperintense tumors within the lungs (3B). Figure 4 represents data collected from mice that received 4T1BR5 cells. 19F signal was observed in brain images acquired at 9.4T (4A) and this signal corresponded to brain metastases detected in 1H images obtained at 3T using a dedicated mouse head RF coil (4B). Brains were sectioned with image guidance, stained with H&E and whole sections were scanned to visualize metastases (4C&D). Adjacent slices were visualized with fluorescence microscopy. Figures 4E&F show the metastases at higher magnification; the PFC is localized to the periphery of the brain metastasis. Lymph node and lung metastases were also stained with F4/80-FITC to detect macrophages. Figure 5 shows the correspondence between 19F MRI, F4/80 (green) and PFC (red) for nodes and lung metastases. In the lymph nodes there are both PFC – and PFC+ macrophages and in the lung metastasis, F480+/PFC+ cells are located along the outer rim of the tumor similar to the spatial distribution seen in the primary breast tumor.Conclusion
This work represents the first time MAMs have been visualized by 19F MRI. MAMs were detected within breast cancer metastases in the lymph nodes, lungs and brain. The ability to detect, quantify and track MAMs in vivo will allow for study of important, unanswered questions about MAMs and the tumor microenvironment, including how MAMs influence metastasis and when during cancer progression MAMs infiltrate tumors.Acknowledgements
No acknowledgement found.References
1. Kelly PM, Davison RS, Bliss E, McGee JO. Macrophages in human breast disease: a quantitative immunohistochemical study. Br J Cancer. 1988;57(2):174-177.
2. Kitamura, T., B.Z. Qian, D. Soong, L. Cassetta, R. Noy, G. Sugano, Y. Kato, J. Li, and J.W. Pollard. 2015. CCL2induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis associated macrophages. J. Exp. Med. 212:1043–1059. http://dx.doi .org/10.1084/jem.20141836
Figures