3077
Selective acidification and de-energization of melanoma xenografts and sensitization to temozolomide1University of Pennsylvania, Philadelphia, PA, United States, 2Thomas Jefferson University, Philadelphia, PA, United States
Synopsis
Using 31P magnetic resonance spectroscopy, we have shown that LND selectively lowers the intracellular pH and decreases ATP levels in human melanoma xenografts. Tumor acidification results from inhibition of lactate export via the monocarboxylic acid transporters and inhibition of pyruvate transport and oxidation via the mitochondrial pyruvate carrier. Energetics is further attenuated by inhibition of electron transport at complex II. Under these conditions, temozolomide accumulates in the tumor as a result of decreases in intracellular pH, which inhibits DNA repair by O6-alkyltransferase via conversion to dacarbazine and formation of diazomethane, and also inhibits glutathione-S-transferase that deactivates the reactive alkylating intermediate.
TARGET AUDIENCE
Investigators interested in animal models of cancer, 1H and 31P MRS, Seahorse analysis, tumor microenvironment, and potentiation of chemotherapy.INTRODUCTION
This study investigates changes in metabolism in DB-1 and WM983B melanomas in response to treatment with lonidamine (LND, an antineoplastic drug that inhibits transmembrane monocarboxylate transporters (MCTs), the mitochondrial pyruvate carrier (MPC) and complex II of electron transport chain.1-6 DB-1 and WM983B are established human melanoma cell lines that express the V600D, E, K, BRAF mutation.7 Using 31P and 1H magnetic resonance spectroscopy (MRS), we detected changes in intracellular pH (pHi), bioenergetics (βNTP/Pi), and lactate concentration of melanoma xenografts in response to treatment with LND indicate that LND potentiates the activity of temozolomide (TMZ) by increasing drug uptake, inhibiting glutathione scavenger activity and DNA repair; these results are supported by initial investigations of DB-1 melanoma xenografts. In the future, treatment with LND plus TMZ could be combined with treatment with targeted mutated BRAF inhibitors and immune checkpoint inhibitors.METHODS
DB-1 and WM983B melanoma cells were grown as described previously.6 In vitro oxygen consumption and extracellular acidification rates for DB-1 and WM983B cells were determined using the Seahorse XF-96 Extracellular Flux Analyzer with and without LND treatment. Glucose and lactate concentrations were measured in both cell lines under the same conditions using a YSI 2300 STAT Plus Glucose & Lactate Analyzer. One million DB-1 and WM983B cells were inoculated subcutaneously (s.c.) into each mouse in 0.1 mL suspensions. MR experiments were performed on a 9.4 T/31 cm horizontal bore Varian spectrometer. When the tumor reached 7-10 mm in diameter along the longest axis, 31P and 1H MRS experiments were performed after positioning the s.c. tumor in a dual-frequency slotted-tube resonator; the pHi, extracellular pH (pHe), βNTP/Pi, and lactate concentration were measured after LND (100 mg/kg; i.p.) administration. Physiological monitoring was maintained during the experiment. Procedures for data acquisition, post processing and parameter estimation were performed as previously described.4-6 Treatment response of DB-1 xenografts to TMZ and LND was measured by tumor growth delay analysis; four cohorts of five age- and weight-matched animals were randomized to the following treatment groups: cohort 1 (sham treated control) was infused intravenously (i.v.) with PBS and given appropriate sham intraperitoneal (i.p.) injections of tris/glycine buffer; cohort 2 was infused i.v. with PBS 40 min after LND administration (100 mg/kg, i.p.); cohort 3 was injected i.p. with tris/glycine buffer and infused i.v. with TMZ (18 mg/kg) in PBS; cohort 4 was injected i.p. with LND and after 40 min (time required for maximum tumor acidification) TMZ (18 mg/kg) was infused i.v. Growth delay data analysis and cell kill estimation were performed as previously described.7 Analysis of variance was performed with Tukey adjustment for multiple comparisons and post-hoc t-tests.RESULTS
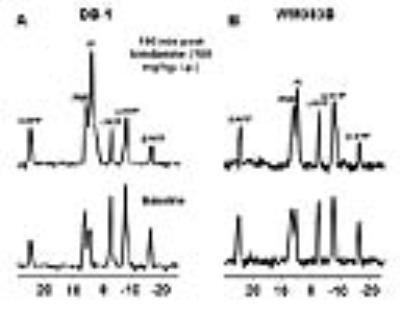
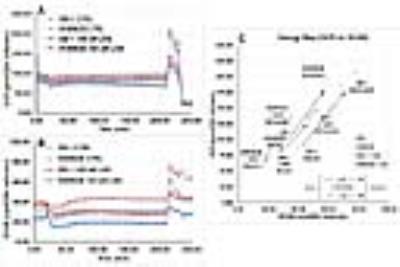
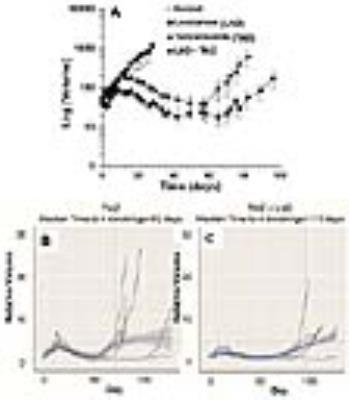
Representative localized 31P MR spectra of DB-1 and WM983B human melanoma xenografts before and after LND treatment are shown in figure 1. Figure 2 shows changes in pHi, pHe, bioenergetics and lactate in response to LND. Figure 3 and table 1 summarize Seahorse data on the metabolic characteristics of DB-1 and WM983B melanoma cells with and without LND treatment. These data show that DB-1 is more glycolytic, LND inhibits mitochondrial capacity of both cell lines but to a smaller extent for the more glycolytic DB-1 line. The effect of treatment with LND + TMZ was evaluated by in vivo tumor growth delay experiments in DB-1 melanoma xenografts (Figure 4). For LND + TMZ, the median time to regrowth (4 doublings) was 113 days compared to 82 days for TMZ alone (Figure 4). 100% of the melanomas (5 animals) responded to treatment with TMZ or LND + TMZ, 20% were cured in both treatment regimens, and 20% in the TMZ regimen exhibited long-term response with recurrence.DISCUSSION AND CONCLUSION
The 31P MR spectra demonstrate that LND leads to intracellular acidification and bioenergetics depression of both lines with more pronounced effects on in vivo DB-1 melanoma xenografts; these parameters are critical indices for tumor response to TMZ. The mechanism of LND potentiation of TMZ via conversion into dacarbazine and formation of diazomethane results from decreases in pHi, which inhibits DNA repair by O6-alkyltransferase and also inhibits glutathione-S-transferase that deactivates the reactive alkylating intermediate. However, TMZ also induces energy-dependent multi-drug resistance that is inhibited by tumor de-energization. Hence, the response to LND + TMZ depends on both the glycolytic and oxidative capacities of the tumors. LND may also increase tumor oxygenation and enhance the effects of reactive oxygen species.Acknowledgements
Support for this project was provided by NIH grants R01-CA129544 and R01-CA172820.References
1. Guo L, Shestov AA, Worth AJ, et al. Inhibition of mitochondrial complex II by the anti-cancer agent lonidamine. J Biol Chem. 2016;291(1):42-57.
2. Nancolas B, Guo L, Zhou R, et al. The anti-tumour agent lonidamine is a potent inhibitor of the mitochondrial pyruvate carrier and plasma membrane monocarboxylate transporters. Biochem J. 2016 Apr 1;473(7):929-36.
3. Nath K, Guo L, Nancolas B, et al. Mechanism of antineoplastic activity of lonidamine. Biochim Biophys Acta. 2016;1866(2):151-62.
4. Nath K, Nelson DS, Heitjan DF, et al. Lonidamine induces intracellular tumor acidification and ATP depletion in breast, prostate and ovarian cancer xenografts and potentiates response to doxorubicin. NMR Biomed. 2015;28(3):281-90.
5. Nath K, Nelson DS, Heitjan DF, et al. Effects of hyperglycemia on lonidamine-induced acidification and de-energization of human melanoma xenografts and sensitization to melphalan. NMR Biomed. 2015;28(3):395-403.
6. Nath K, Nelson DS, Ho AM, et al. 31P and 1H MRS of DB-1 melanoma xenografts: lonidamine selectively decreases tumor intracellular pH and energy status and sensitizes tumors to melphalan. NMR Biomed. 2013;26(1):98-105. PMCID: 3465621.
7. Nath K, Nelson DS, Putt ME, et al. Comparison of the Lonidamine Potentiated Effect of Nitrogen Mustard Alkylating Agents on the Systemic Treatment of DB-1 Human Melanoma Xenografts in Mice. PLoS One. 2016;11(6):e0157125.
Figures