3076
Non-invasive imaging of macrophage accumulation in abdominal aortic aneurysm by 19F MRI1Molecular Cardiology, University of Düsseldorf, Düsseldorf, Germany, 2Department of Internal Medicine/Nephrology, University Hospital Düsseldorf, Düsseldorf, Germany, 3Division of Cardiology, Pulmonology and Vascular Medicine, University of Düsseldorf, Düsseldorf, Germany
Synopsis
Abdominal aortic aneurysms (AAA) are a relatively common disease, with still unclear etiology that is associated with high mortality due to aortic rupture. Here we show that mice deficient for the Mas-receptor show aggravated AAA formation upon Ang-II treatment and that accumulation of macrophages in bulk aneurysms can be non-invasively visualized by 1H/19F MRI using intravenously applied perfluorocarbon nanoemulsions which are efficiently phagocytosed by macrophages. We conclude that Mas-receptor deficiency leads to increased inflammation with enhanced AAA formation and that 19F MRI-based inflammation imaging will help to further unravel the role of monocytes/macrophages in the course of AAA progression.
Background
Abdominal aortic aneurysms (AAA) are a relatively common disease which is associated with high mortality due to aortic rupture (1). The precise biological reasons for the formation and progression of aneurysms are still unclear, but there is evidence that hypertension induced by the renin-angiotensin system as well as inflammatory processes are important factors. Recently, it has been recognized that the angiotensin/Mas receptor pathway is a major counterbalancing mechanism of the renin-angiotensin system and that effects triggered by the Mas receptor (MasR) are crucial to attenuate the inflammatory and remodeling processes in different pathophysiological conditions.
Several imaging modalities are utilized to diagnose and monitor AAAs, however imaging the inflammatory component of AAAs is still difficult. Our group has recently utilized intravenously injected perfluorocarbon nanoemulsions (PFC) - which are phagocytosed by monocytes and macrophages - to unequivocally visualize inflammation in a variety of clinically relevant disease models by combined 1H/19F MRI (2-5). Due to the absence of any 19F background in biological tissue, these 19F signals are highly specific and the combination with anatomical 1H images enables a precise localization of inflammatory hot-spots. In the present study we aimed (i) to apply this approach for monitoring of monocyte/macrophage infiltration during development of AAAs and (ii) to investigate the consequences of a MasR depletion on AAA formation by 19F MRI-based inflammation imaging.
Methods
ApoE-/- and ApoE-/-/MasR-/- mice (fed with normal diet) were treated with angiotensin II (1000 ng/kg/min) via subcutaneously implanted osmotic minipumps for 28 days and monitored longitudinally by 1H/19F MRI at 9.4T using a microimaging unit with actively shielded 40-mm gradients (1.5 T/m maximum gradient strength, 110 µs rise time at 100 % gradient switching). Mice were placed in a 25-mm 1H/19F birdcage resonator and after acquisition of anatomical 1H reference images, MR angiography was performed using a flow compensated 2D flash sequence (256x192 matrix size; 0.5 mm slice thickness, 0.25 mm overlap; scan time 3 min; 80° flip angle).
To monitor macrophage infiltration, PFC were injected on day 4 and the 1H/19F MRI datasets were acquired 72 h later, to ensure optimal accumulation of PFC-loaded cells at inflammatory foci. For 19F imaging, a 19F RARE sequence with the following parameters was applied: 2.56´2.56 cm2 FoV, 64×64 matrix, 1 mm slice thickness, TR 4000 ms, RARE factor 32, 256 averages, 34 min acquisition time. Ex vivo high resolution 1H/19F MRI scans were performed using a 3D RARE sequence with the following parameter: 1.0´1.0´3.0 cm3 FoV, 128×128×192 matrix (19F = 64×64×96), TR 2500 ms, RARE factor 32, 8 averages (19F=180), 5 h (19F = 14 h) acquisition time.
Results and Discussion
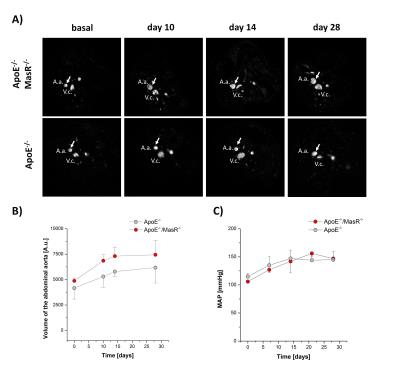
1H MR angiography clearly revealed that Ang-II-induced hypertension led an increased lumen diameter of abdominal aorta in the suprarenal area of both ApoE-/- and ApoE-/-/MasR-/- mice within 28 days (Fig. 1A/B). However, this effect was more pronounced in ApoE-/-/MasR-/- compared to ApoE-/- mice (Fig. 1B), although the arterial blood pressure was similar in both mouse strains (Fig. 1C). The increased volume load of the aorta was also associated with a more frequent appearance of bulk aneurysms in ApoE-/-/MasR-/- (~ 72%) compared to ApoE-/- mice (~ 40%) and could already be observed by 1H MR angiography on day 7 of the Ang-II treatment.
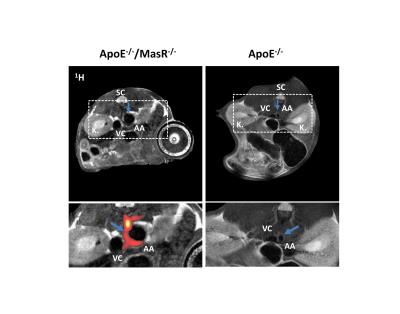
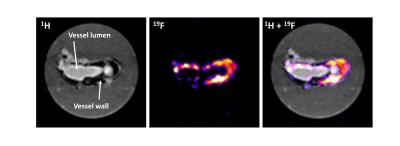
To analyze the accompanying macrophage infiltration into the abdominal aorta, we injected PFCs on day 4 after Ang-II treatment and subjected mice 72 h afterwards to 19F MRI for efficient accumulation of PFC-loaded cells in inflammatory foci. Already at this early time, we found strong 19F signals surrounding the abdominal aorta in ApoE-/-/MasR-/-, however only in those areas with bulk aneurysms (Fig. 2A). The presence of 19F signals - which indicates macrophage infiltration into the injured vessel wall - was corroborated by high resolution ex vivo 1H/19F MRI of isolated and fixed abdominal aortae (Fig. 3). Finally, histological analysis confirmed the presence of large amounts of macrophages within this area.
Conclusions
The data of the present study demonstrate that 1H/19F MRI is suitable to visualize the inflammatory component of AAA, which may be exploited to further elucidate the role of macrophages in AAA and to monitor the effects of novel therapeutic concepts for AAA. In this context, we show that lack of the MasR increases inflammation and macrophage infiltration in AAA which is associated with an enhanced progression of the disease.Acknowledgements
No acknowledgement found.References
1: Daugherty A, Cassis LA, Lu H. Complex pathologies of angiotensin II-induced abdominal aortic aneurysms. J Zhejiang Univ Sci B. 2011 Aug;12(8):624-8.
2: Flögel U, Ding Z, Hardung H, Jander S, Reichmann G, Jacoby C, Schubert R, Schrader J. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation. 2008 Jul 8;118(2):140-8.
3: Ebner B, Behm P, Jacoby C, Burghoff S, French BA, Schrader J, Flögel U. Early assessment of pulmonary inflammation by 19F MRI in vivo. Circ Cardiovasc Imaging. 2010 Mar;3(2):202-10.
4: Flögel U, Burghoff S, van Lent PL, Temme S, Galbarz L, Ding Z, El-Tayeb A, Huels S, Bönner F, Borg N, Jacoby C, Müller CE, van den Berg WB, Schrader J. Selective activation of adenosine A2A receptors on immune cells by a CD73-dependent prodrug suppresses joint inflammation in experimental rheumatoid arthritis. Sci Transl Med. 2012 Aug 8;4(146):146ra108.
5: Temme S, Bönner F, Schrader J, Flögel U. 19F magnetic resonance imaging of endogenous macrophages in inflammation. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012 May-Jun;4(3):329-43.
Figures