3075
19F MR-Imaging of acute thrombi using a clinically relevant perfluorocarbon nanoemulsion1Molecular Cardiology, University of Düsseldorf, Düsseldorf, Germany, 2Division of Cardiology, Pulmonology and Vascular Medicine, University of Düsseldorf, Düsseldorf, Germany, 3Pharmaceutical Technology and Biopharmacy, University of Freiburg, Freiburg i. Br.
Synopsis
Exact localization of acute thrombi is still a serious problem in the clinical setting. Here we show the feasibility of artefact-free 19F MR-imaging using clinically relevant PFOB-nanoemulsions functionalized with an α2-antiplasmin peptide, specific for early thrombi. Utilizing the isolated CF3-peak of PFOB in combination with a conventional 19F RARE sequence is suitable for specific and artefact-free imaging of acute thrombi in vitro and also in vivo.
Background
Exact localization of acute thrombi is still a serious problem in the clinical setting and has significant implications for antithrombotic treatment regiments, since chronic thrombi are resistant towards thrombolysis. Detection of thrombi by conventional 1H MR methods, like angiography or T1/T2-weighted techniques can be challenging, inparticular if small, non-occlusive thrombi have only minor impact on blood flow and therefore may not be detected in weighted images.
To overcome this limitation, we have recently used 19F MRI for detection of early thrombi utilizing a targeted perfluorocarbon nanoemulsion (perfluoro-15-crown-5 ether = PFCE) functionalized with a peptide derived from α2-antiplasmin (α2AP) which specifically accumulates in early thrombi (1). Due to the fact that 19F is nearly absent from biological tissue, this appraoch enabled the unequivocal detection of early thrombi without any background signal.
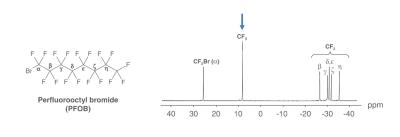
PFCE is advantageous for imaging purposes due to its 20 magnetically identical 19F atoms, but has an extremly long biological half-life and is therefore not suitable for any clinical application (2). An alternative is perfluorooctyl bromide (PFOB) which has a more complex spectrum but – due to its low vapor pressure – has a short tissue retention time (2). PFOB has also been used in clinical trials as blood substitute. Normally, complex 19F spectra are not suitable for conventional 19F imaging sequences due to the occurence of chemical shift artefacts. However, the CF3-peak within the 19F spectrum of PFOB is clearly separated might suitable for artefact-free 19F MRI imaging using a conventional RARE sequence (Fig.1).
In the present study, we aimed to investigate whether the combination of α2AP targeted PFOB emulsions with imaging of the CF3-peak by a conventional 19F RARE sequence is suitable for the detection of acute thrombi by 19F MRI.
Methods
Using the semifluorinated diblock F6H10 as stabilizer, we generated stable PFOB nanoemulsions with a high fluorine content (40% w/w). To generate targeting PFCs, we used a 14 aa peptide derived from α2-antiplasmin (α2AP) which is cross-linked to the developing fibrin network by the active factor XIII (restricted to the very early phase of the thrombus formation). First, α2AP as well as Q3A-peptides (with 10-fold lower affinity for FXIIIa) were coupled to a maleimide-chlesterol-PEG2000-anchor. Resulting α2AP/Q3A-PEG2000-cholesterol conjugates were incubated with preformed PFOB-emulsions which leads to the spontaneous insertion of the cholesterol moiety into the lipid layer of the PFC (sterol-based post-insertion = SPIT).
Ex vivo experiments were carried out with thrombi obtained by platelet rich plasma of human blood which were incubated with targeted PFCs about 5 min after induction of thrombus formation. For in vivo experiments, a murine model of deep venous thrombosis was was applied which resulted in non-occlusive thrombi. Combined 1H/19F MRI was performed at a 9.4T Bruker AvanceIII WideBore NMR spectrometer using a microimaging unit (Micro 2.5) with actively shielded 40-mm gradients (1 T/m maximum gradient strength, 110 µs rise time at 100 % gradient switching). Mice were placed in a 25-mm 1H/19F birdcage resonator and after acquisition of anatomical 1H reference images, a 19F RARE sequence with the following parameters was used: 2.56´2.56 cm2 FoV, 64×64 matrix, 1 mm slice thickness, TR 4000 ms, RARE factor 32, 256 averages, 34 min acquisition time.
Results and Discussion
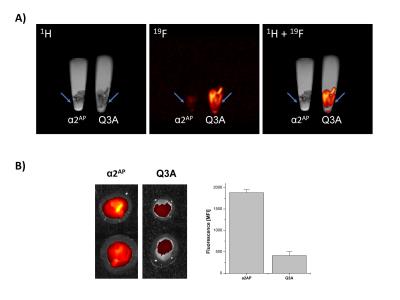
To test the suitability of these emulsion for thrombus-labelling, we first incubated the α2AP-/Q3A-emulsions with acute thrombi derived from human platelet rich plasma and analyzed the samples by fluorecence imaging and 19F MRI. For 19F MRI, we utilized a conventional RARE sequence applied to the CF3-group of the PFOB. To prevent any chemical-shift artefacts by the neighbouring CF2-Br peak due to line-broadening in vivo, we reduced the excitation bandwidth to 10.000 Hz. Imaging of α2AP-/Q3A-PFOB labelled ex vivo generated human thrombi from several volunteers revealed a artefact-free 19F images in the α2AP-labelled thrombi, whereas the thrombi incubated with Q3A-PFOB showed only low signal (Fig. 2A). This was also reflected by measurement of the fluorescence signal (Fig.2B), showing a specific accumulation of α2AP-PFOBs in acute thrombi. In contrast, chronic thrombi were neither labelled with α2AP-PFOB no Q3A-PFOB.
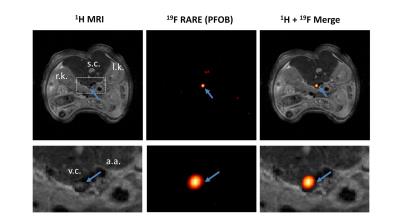
To validate this approach also in vivo, we used a model of acute deep venous thrombosis, by induction of vessel injury due to topical application of FeCl3. Intravenous injection of α2AP-PFOB emulsions shortly before thrombus induction resulted in a highly specific artefact-free 19F signals in acute thrombi without any 19F background from surrounding tissue.
Conclusions
Stable α2AP-PFOB emulsions, generated by sterol-based post-insertion in combination with a conventional RARE sequence and a narrow bandwith excitation pulse are suitable for artefact-free imaging of acute thrombi by 19F MRI.Acknowledgements
No acknowledgement found.References
1: Temme S, Grapentin C, Quast C, Jacoby C, Grandoch M, Ding Z, Owenier C, Mayenfels F, Fischer JW, Schubert R, Schrader J, Flögel U. Noninvasive Imaging of Early Venous Thrombosis by 19F Magnetic Resonance Imaging With Targeted Perfluorocarbon Nanoemulsions. Circulation. 2015 Apr 21;131(16):1405-14.
2: Jacoby C, Temme S, Mayenfels F, Benoit N, Krafft MP, Schubert R, Schrader J, Flögel U. Probing different perfluorocarbons for in vivo inflammation imaging by 19F MRI: image reconstruction, biological half-lives and sensitivity. NMR Biomed. 2014 Mar;27(3):261-71.
Figures