3074
ROS imaging by endogenous contrast MRI: specificity and translational premises1Radiology, College of Medicine, University of Illinois at Chicago, Chicago, IL, United States, 2Bioengineering, University of Illinois at Chicago, Chicago, IL, United States, 33T Research Program, Center for MR Research, College of Medicine, University of Illinois at Chicago, Chicago, IL, United States, 4Research Resource Center, College of Medicine, University of Illinois at Chicago, Chicago, IL, United States, 5Radiology, College of Medicine, Northwestern University, Chicago, IL, United States
Synopsis
Detection of the elusive Reactive Oxygen Species is pivotal for understanding and diagnosis of many diseases. Recently ROS has been imaged with endogenous MRI contrasts in biological system by reduction in CEST trough combined T1 shortening and proton exchange enhancing effects. We here show potential confounding factors such as H2O2, molecular oxygen, iron oxidation, pH, and temperature to be negligible in phantom studies. In addition, for the first time, we have imaged ROS by endogenous MRI contrasts under physiological conditions, at clinical MRI field strength and on ex vivo brain tissue, paving the path for clinical translation.
BACKGROUND and PURPOSE
Detection of reactive oxygen species (ROS) can provide a new avenue to understanding disease mechanisms and diagnosing a wide spectrum of pathologies. Unfortunately, ROS exhibit a marked elusive nature, due to low concentrations (below mmol) and short lifetimes (in μs range) in vivo1,2. To date, electron paramagnetic resonance and fluorescence are the main strategies adopted to detect and measure ROS but both rely on targeted probes or exogenous contrast agents3. Recently a fully noninvasive and endogenous MRI-based technique was proposed to image ROS in H2O2-treated fresh egg white phantoms down to pM concentrations4-6, characterized by reduced T1 and increased proton exchange rate, leading to reduced overall chemical exchange saturation transfer (CEST) contrast. Besides the high sensitivity, specificity issues remain to be addressed as the measurement may be contaminated by some chemical and physical confounding factors that may change during the ROS-producing Fenton reactions7. The purpose of this study is to investigate such contributions and to assess the feasibility of our approach at physiological conditions, clinical MRI field strength and on ex vivo tissue.METHODS
H2O2 itself, iron oxidation and molecular oxygen, together with temperature and pH, may act as confounding factors for the observed contrasts from ROS. To avoid ROS production7, the specificity imaging studies were then performed on three sets of pure bovine serum albumin (BSA) solutions containing respectively: different concentrations of H2O2, 1 mmol FeCl2 and FeCl3, and molecular oxygen introduced by bubbling. Also, egg white phantoms were prepared with different concentrations of H2O2 and temperature and pH were monitored up to completion of reaction.
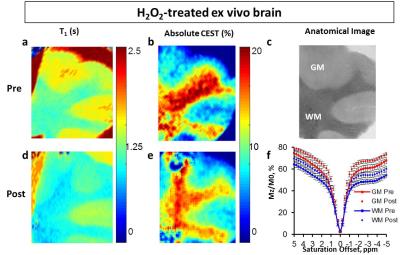
In order to test the feasibility at physiological conditions, imaging was performed on samples at 37°C and at neutral pH. An additional egg white set at pH 7 with different concentrations of H2O2 was scanned at 3T to test the translation of the protocol at a clinical field. Finally, to further test the protocol translation, ex vivo lamb brain tissue was treated with 0.025 v/v% H2O2 for 1 hour and imaged before and after treatment.MRI experiments at 9.4T included T1, T2, and B0 mapping and CEST (Z-spectra from -5 to +5ppm, 3s square saturation pulse of 1.17 µT, contrast at 3.5 ppm). Exchange rate was computed by fitting with Bloch-McConnell equations QUESP data (QUantification of Exchange using Saturation Power)9.
RESULTS and DISCUSSION
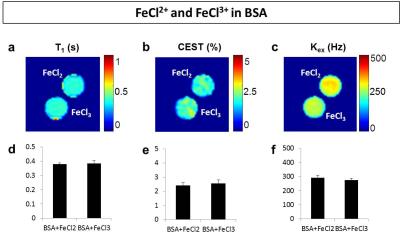
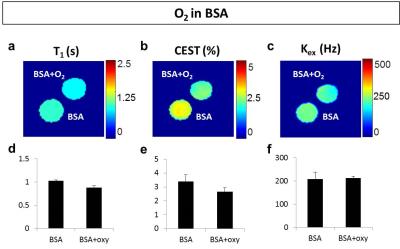
Addition of H2O2 alone didn’t generate changes in T1, but decreased T2 and increased CEST8, which is different from ROS induced contrasts as reported in previous studies4-6. Treatment with iron chlorides showed no significant differences between Fe2+ and Fe3+ solutions (Fig. 1). Molecular oxygen slightly reduced T1 and CEST (by 10%) compared to the control, but exchange rate was found on average unvaried (Fig. 2). In summary, none of these factors delivered the changes in MRI contrasts reported in H2O2-treated egg white experiments4-6. In addition, the Fenton reaction in egg white phantoms didn’t perturb neither temperature (<1°C) nor pH (<0.1), suggesting a minimal contributions from these factors as well.
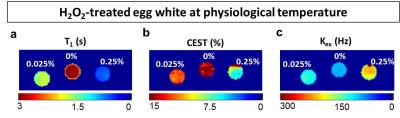
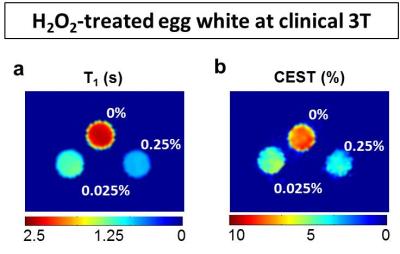
Instead, our egg white studies confirmed the reductions (>75% T1 and >40% CEST) in experiments carried out at body temperature and neutral pH (Fig.3,4). Exchange rate was also increased up to twofold the control value, consistently with previous data4-6 at laboratory conditions. In the experiment performed at 3T, the samples again exhibited reduced CEST (up to more than 50%) and T1 (up to 75%) proportionally to peroxide concentration (Fig. 4). Finally, extracted brain tissue as well showed reduced T1 (20%) and CEST (25% and 38% for white and gray matter) after H2O2 treatment. It’s clear that in vivo clinical conditions may be more complicated than the egg white model and specificity issues may need to be addressed in a case by case means. Nevertheless, in this study, we have addressed the major confounding factors and ruled out their contribution. In addition, we demonstrated that the study can be performed in a clinical setting under physiological conditions as well as on ex vivo tissue. The consistency of the results throughout the different experiments suggests that this method may have tremendous impact on the imaging of ROS.
CONCLUSION
ROS can be imaged with endogenous MRI contrasts in biological system by reduction in CEST trough combined T1 shortening and proton exchange enhancing effects. We have shown potential confounding factors such as H2O2, molecular oxygen, iron oxidation, pH, and temperature to be negligible in phantom studies. In addition, for the first time, we have imaged ROS by endogenous MRI contrasts under physiological conditions, at clinical MRI field strength, and on ex vivo tissue, paving the path for clinical translation.Acknowledgements
No acknowledgement found.References
1. Attri, P., et al. Generation mechanism of hydroxyl radical species and its lifetime prediction during the plasma-initiated ultraviolet (UV) photolysis. Scientific reports 5, 9332 (2015).
2. Grill, H.P., Zweier, J.L., Kuppusamy, P., Weisfeldt, M.L. & Flaherty, J.T. Direct measurement of myocardial free radical generation in an in vivo model: effects of postischemic reperfusion and treatment with human recombinant superoxide dismutase. Journal of the American College of Cardiology 20, 1604-1611 (1992).
3. Emoto, M.C., et al. Brain imaging in methamphetamine-treated mice using a nitroxide contrast agent for EPR imaging of the redox status and a gadolinium contrast agent for MRI observation of blood–brain barrier function. Free Radical Research 49, 1038-1047 (2015).
4. Tain R-W et al.,Oxidative Stress Sensitive Magnetization Transfer. Proceedings 23rd International Conference of the ISMRM; Toronto. (2015)
5. Tain R-W et al., Imaging Oxidative Stress Using CEST MRI. Symposium on Chemical Exchange Saturation Transfer; 2015; Philadelphia, PA.
6. Tain R-W et al., Imaging Reactive Oxygen Species (ROS) using CEST MRI. International Society for Magnetic Resonance in Medicine; 2016; Suntec City, Singapore.
7. Lin, S.-S. & Gurol, M.D. Catalytic Decomposition of Hydrogen Peroxide on Iron Oxide: Kinetics, Mechanism, and Implications. Environmental Science & Technology 32, 1417-1423 (1998).
8. D. Ryoo et al. CEST MRI detection of hydrogen peroxide encapsulated PLGA microcapsules.
9. McMahon MT et al., Quantifying exchange rates in chemical exchange saturation transfer agents using the saturation time and saturation power dependencies of the magnetization transfer effect on the magnetic resonance imaging signal (QUEST and QUESP): Ph calibration for poly-L-lysine and a starburst dendrimer. Magn Reson Med. 2006 Apr;55(4):836-47.
Figures