3072
Compressed Sensing with Signal Averaging Reduces Motion Artifacts in Fluorine-19 MRI1Radiology, University Hospital of Lausanne (CHUV)-University of Lausanne (Unil), Lausanne, Switzerland, 2Center for Biomedical Imaging (CIBM), Lausanne, Geneva, Switzerland, 3Advanced Clinical Imaging Technology (HC CMEA SUI DI PI), Siemens Healthcare AG, Lausanne, Switzerland, 4Signal Processing Laboratory 5 (LTS5), Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland, 5Angiology service, University Hospital of Lausanne (CHUV)-University of Lausanne (Unil), Lausanne, Switzerland
Synopsis
In addition to conventional signal averaging, compressed sensing (CS) can be applied to fluorine-19 MRI to improve its low signal-to-noise ratio. For a given acquisition time and CS algorithm, an N-averages N-fold-undersampled dataset results in higher sensitivity than a fully sampled non-averaged dataset. However, it is still unclear whether averaging changes the sensitivity to motion artifacts for an undersampled acquisition.We therefore tested the hypothesis that an N-averages N-fold undersampled acquisition is more robust against motion artifacts than a fully sampled non-averaged acquisition when both are reconstructed with CS.
Introduction
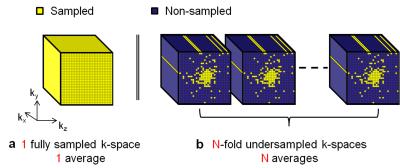
In recent years, fluorine-19 (19F) MRI of injected perfluorocarbon emulsions (PFCs) has been used for inflammation imaging and cell tracking1,2. Since 19F is not naturally abundant in the body, the measured MR signal is directly related to the absorbed concentration of injected PFC, either on the inflammation site or in the liver and spleen mainly. 19F MRI thus allows for a straightforward quantification of the concentration. However, this concentration is relatively low and requires signal averaging to improve the signal-to-noise ratio (SNR), which results in extended scan times. To address this limitation, several studies have investigated the use of compressed sensing (CS)3,4, and it has been demonstrated that the combination of signal averaging with an undersampling regime improves the sensitivity per unit time when reconstructed with CS5. This phenomenon is a result of the higher SNR of the averaged k-space points combined with the variable-density undersampling pattern that keeps the k-space center fully sampled (Figure 1). Another well-known advantage of averaging is a reduced sensitivity to motion artifacts. However, to our knowledge it is still unclear whether and to what degree this advantage is maintained once the acquisition is incoherently undersampled. In this study, we therefore tested the hypothesis that an N-fold undersampled acquisition with N averages is more robust against motion artifacts than a fully sampled non-averaged acquisition, when both are reconstructed with CS.Methods
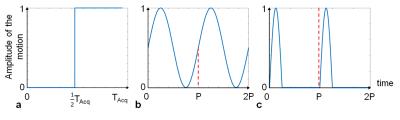
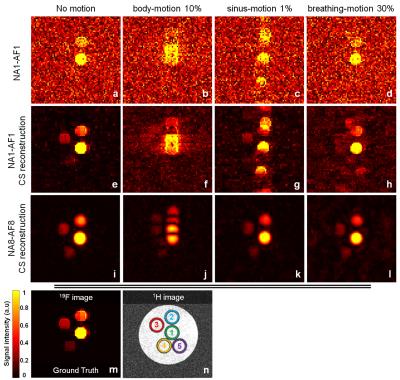
Images were acquired at 3T (Magnetom Prisma, Siemens) with a 35-mm-diameter 19F/1H volume RF coil (Rapid Biomedical), and an isotropic 3D turbo spin echo prototype pulse sequence (matrix size 64x64x64, voxel size 0.5x0.5x0.5mm3, echo train length 10, and TR/TE=847/9.4ms). A phantom was made of 5 syringes of agar gel mixed with PFPE (perfluoropolyether, Celsense Inc) at different 19F concentrations (1.05/0.52/0.26/0.13/0M). Two different acquisitions were performed: a non-averaged, fully sampled acquisition (number of averages NA=1, acceleration factor AF=1; NA1-AF1) and an 8 averages, 8-fold prospectively undersampled acquisition (NA8-AF8), which have an identical acquisition time Tacq=7:09min. Three different simulated motion patterns were applied to both acquisitions: a one-time displacement of the phantom (body-motion, Figure 2a), a regular sinusoidal motion (sinus-motion, Figure 2b) and an asymmetric periodic pattern that simulates respiratory motion with a long end-expiration and a short inspiration period (breathing-motion, Figure 2c). A fully sampled acquisition with 32 averages was obtained and used as ground truth (GT) for the analysis. A previously published CS algorithm6,7 was used to reconstruct all datasets (Matlab), and behaved like a wavelet denoising filter for NA1-AF1. As a qualitative measure of image quality, the number of visible tubes (NTV) in each image was counted and averaged; the 1H image was used as a guide to distinguish real tubes from ghosting artifacts. As a quantitative measure of image quality, a Dice similarity coefficient (DSC)8,9 was calculated between GT and a test image (Test, either NA1-AF1 or NA8-AF8, both with CS reconstruction):
$$DSC(GT, Test) = 2\frac{GT\bigcap Test}{GT + Test}. \qquad (1)$$
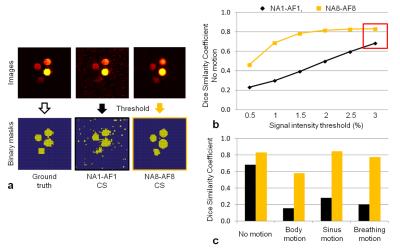
DSC represents the percentage of overlap between two binary masks generated by applying a threshold to GT and Test images; this threshold was calibrated to allow the highest DSC without suppressing signal intensity information from the four tubes in the GT binary mask. DSC was used to assess the differences (paired Student’s t-test) between the tested protocols in the different motion scenarios. To confirm the results in vivo with true breathing motion, both NA1-AF1 and NA8-AF8 protocols were acquired in two mice that received intraperitoneal injections of 300μl of PFPE 24h before imaging.
Results and Discussion
NA1-AF1 images with and without CS visually exhibited a higher degree of motion distortion than NA8-AF8 images (Figure 3). This is particularly significant for the sinus- and breathing-motion regimes, where there were no ghosting artifacts in the NA8-AF8 images. Qualitatively, NA8-AF8 with CS consistently scored better than the NA1-AF1 with and without CS reconstruction (NVT=3.75 vs NVT=2.5 vs NVT=1.5, respectively). Without motion, NA8-AF8 had a higher DSC than NA1-AF1 for all threshold levels (p<0.001, Figure 4a,b). For the chosen threshold (3% of maximum signal intensity in GT image), NA8-AF8 resulted in higher DSC than NA1-AF1 for all motion patterns (p=0.02, Figure 4c). In vivo, the PFC-loaded liver is visible in the NA1-AF1 and NA8-AF8 images with CS, while it is not distinguishable from the noise in NA1-AF1 without CS. The delineation of the liver in the NA8-AF8 image was furthermore sharper than in the NA1-AF1 image (Figure 5).Conclusion
We validated the hypothesis that an N-fold undersampled acquisition with N averages is more robust against motion artifacts than a fully sampled non-averaged acquisition, when both are reconstructed with CS.Acknowledgements
This work was supported by grants from the Pierre Mercier Foundation, the Swiss Multiple Sclerosis Society and the Swiss National Science Foundation (PZ00P3-154719) to RBvH, as well as by the Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL, and the Leenaards and Jeantet Foundations.References
1.Ruiz-Cabello et al. Fluorine (19F) MRS and MRI in Biomedicine. NMR Biomed. 2010; 24(2):114-29.
2.Ahrens et al. In Vivo MRI Cell Tracking Using Perfluorocarbon Probes and Fluorine-19 Detection. NMR Biomed. 2013; 26(7):860-71.
3.Kampf et al. Application of Compressed Sensing to In Vivo 3D 19F CSI. J Magn Res. 2010; 207(2):262-73.
4.Zhong et al. Accelerated Fluorine-19 MRI Cell Tracking Using Compressed Sensing. Magn Reson Med. 2012; 69(6):1683-90.
5.Lugand et al. Can Signal Averaging Combined with Undersampling and Compressed Sensing Improve Sensitivity?. Proc Soc Magn Reson Angio. 2016; 12.
6.Lustig et al. Sparse MRI: The Application of Compressed Sensing for Rapid MR Imaging. Magn Reson Med. 2007; 58(6):1182-95.
7.Yerly et al. Coronary Endothelial Function Assessment Using Self-Gated Cardiac Cine MRI and k-t Sparse SENSE. Magn Reson Med. 2016; 76(5):1443-1454.
8.Dice. Measures of the Amount of Ecologic Association Between Species. Ecology. 1945; 26: 297-302.
9.Zhang et al. Effect of Compressed Sensing Reconstruction on Target and Organ Delineation in Cone-Beam CT of Head-and-Neck and Breast Cancer. Radiother Oncol. 2014; 112(3):413-7.
Figures