3071
Metastatic Liver Cancer Targeted Liposomal Theranostic Prodrug for in vivo Diagnosis and Therapy1Bioimaging Research Team, Korea Basic Science Institute, Cheongju-Si, Korea, Republic of
Synopsis
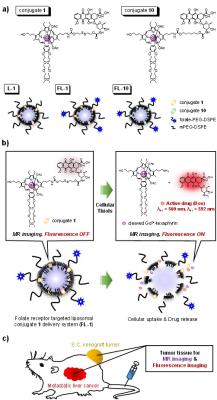
Reported here is a new theranostic agent, 1, which consists of a Gd3+-texaphyrin core conjugated to a doxorubicin prodrug via a disulfide bond undergoing cleavage in the presence of glutathione, a species typically upregulated in cancer cells. To improve the solubility and tumor targeting of 1, it was loaded into folate receptor-targeted liposomes to produce FL-1. FL-1 was found to selectively produce a greater anti-proliferative effect in the case of the KB and CT26 cell lines as compared to the HepG2 and NIH3T3 cell lines. FL-1 was also found to provide enhanced MR imaging in vivo under conditions of T1 contrast in the early stage of metastatic cancer progression.
Introduction
The early diagnosis and accurate characterization of metastatic lesions is crucial to determining the prognosis of the patient and making proper therapeutic decisions. We believe that the combination of MR and fluorescence imaging could provide synergistic advantages over either modality alone. Here, we report an example of such a dual detection theranostic agent. It is based on a combination of a gadolinium (Gd3+) texaphyrin and a doxorubicin cytotoxin. This agent allows liver cancer imaging in both subcutaneous and metastatic liver cancer murine models while reducing the cancer burden as inferred from tumor regrowth studies.Methods
With the above goals in mind, we have designed the new theranostic agent 1, which consists of a Gd3+-texaphyrin core conjugated to a doxorubicin prodrug via a disulfide bond. Conjugate 1 was designed to undergo cleavage in the presence of glutathione (GSH), a species typically upregulated in cancer cells. To improve the solubility and enhance the tumor targeting of 1, it was loaded into folate receptor-targeted liposomes to produce FL-1 (for folate liposome loaded with 1), as illustrated in Figure 1. To test this hypothesis, a variety of in vitro and in vivo studies were carried out using the KB and CT26 (folate receptor positive) and HepG2 and NIH3T3 (folate receptor negative) cell lines.Results
Characteristics of FL-1: Evidence of GSH-induced cleavage came from fluorescence studies carried out in PBS (pH 7.4) at 37 °C. FL-1 displays a very weak fluorescence. However, when exposed to excess GSH (up to 1500 equiv) a ca. 38-fold increase in the fluorescence intensity at 592 nm is observed. The T1 relaxivities of FL-1 were calculated to be 11.8 ± 0.3 and 7.1 ± 0.4 mM-1s-1 at 60 and 200 MHz, respectively. Phantom images acquired at 200 MHz in PBS reveal increasingly bright signals as the concentration of FL-1 increases.
In vitro anticancer effect: The in vitro anticancer effects of FL-1 were examined using standard MTT cell viability assays. Significant and moderate anti-proliferative activity was seen at 5 μM in the case of the KB and CT26 cell lines, respectively. Dose dependent effects were seen, with the activity increasing with concentration. At all concentrations, the activity was lower in the case of the HepG2 and NIH3T3 cell lines lacking the over-expressed folate receptors present in the KB and CT26 cell lines.
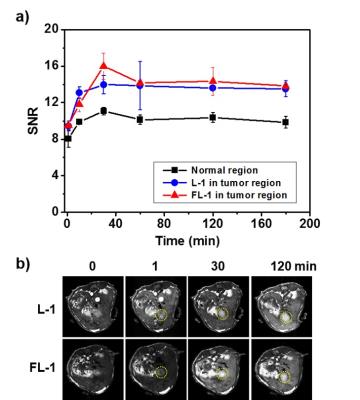
In vivo theranostic effect: The metastatic liver cancer model used for this study was obtained via the intrasplenic administration of CT26 cells to nude mice. Enhanced MR signals ascribed to FL-1 were seen in the tumor region as early as 30 min after i.v. administration (tail vein injection). This stands in contrast to what was seen in the case of L-1. The intensity of the signal decreased only gradually with time, presumably reflecting slow clearance of the conjugate from the tumor site (Figure 2a). As inferred from T1-weighted MR images (Figure 2b), FL-1 reveals effectively the tumor area, which is surrounded by normal liver tissue, and can do so at an early stage of metastatic disease (day 3 post-inoculation). The survival rates of the saline control, the FL-10 control, and the FL-1-treated group were compared using the metastatic liver model mice. No mice treated with saline survived past day 45. On the other hand, at that time (i.e., day 45 post inoculation) 37.5% of the mice treated with FL-1 were still alive. By day 60 none of the FL-10 treated animals were alive, whereas several treated with FL-1 were.
Discussions and Conclusions
We have described the synthesis, spectroscopic properties, target-specific internalization, and therapeutic effects of conjugate 1 and the liposomal formulation FL-1, as theranostic agents. The use of folate receptor-targeting liposomes permits enhanced tumor uptake. GSH-mediated cleavage of the disulfide linker leads to release of free doxorubicin as well as a fluorescence increase at 592 nm. Enhanced imaging, as well as anti-proliferative effects, were seen in the folate-receptor positive cell lines, KB and CT26, relative to the folate-receptor negative cell lines, HepG2 and NIH3T3. The present system, FL-1, permits enhanced MR imaging in vivo under conditions of T1 contrast, allowing the progression of metastatic cancer to be followed in vivo during its early stages. On the basis of the findings presented here we propose that conjugates, such as 1 and its liposomal formulation FL-1, that permit dual imaging and which provide a therapeutic potential may have an important role to play as theranostics, particularly for the early diagnosis and treatment of metastatic liver cancer.Acknowledgements
This work was supported by a grant from Korea Basic Science Institute (D36401, K.S.H.).References
No reference found.Figures