3070
A Specific MRI contrast agent for glioma: Targeted Quadruple Mutant IL-13 (TQM-13) conjugated liposomes encapsulated with Magnevist@1Radiology, College of Medicine Penn State University, Hershey, PA, United States, 2Neurosurgery, College of Medicine, Penn State University, Hershey, 3Radiology and Nuclear Medicine Wake Forest School of Medicine, NC, USA
Synopsis
There is a clinical need for targeted MRI-contrast agents that are more sensitive and specific in detection of glioma than conventional MRI contrast. We use interleukin-13 (IL-13) as targeting ligand because 75% of glioma cells overexpress IL-13Rα2 significantly1. We investigated the relative efficacy of liposomes conjugated with wild type IL-13 to a variant of IL-13, known as Targeted Quadruple Mutant13 (TQM-13) that has been shown to be more selective for the IL-13Rα2 and binds with higher affinity than the wide type. Our targeted MRI agent, TQM-13-liposomes-Gd, produced specific MRI contrast, delineating tumor, inflamed and normal tissues.
synopsis: There is a clinical need for targeted MRI-contrast agents that are more sensitive and specific in detection of glioma than conventional MRI contrast. We use interleukin-13 (IL-13) as targeting ligand because 75% of glioma cells overexpress IL-13Rα2 significantly1. We investigated the relative efficacy of liposomes conjugated with wild type IL-13 to a variant of IL-13, known as Targeted Quadruple Mutant13 (TQM-13) that has been shown to be more selective for the IL-13Rα2 and binds with higher affinity than the wide type. Our targeted MRI agent, TQM-13-liposomes-Gd, produced specific MRI contrast, delineating tumor, inflamed and normal tissues.
Introduction: There is a clinical need for targeted MRI-contrast agents that are more sensitive and specific in detection of glioma than conventional MRI contrast such as Magnevist. The mechanism for tumor detection with a conventional contrast enhanced MRI is that the contrast agents extravagate into the brain areas due to breakdown of the blood brain barrier (BBB) by the tumor. However, glioblastoma multiform (GBM) aggressively infiltrates into the brain tissue where BBB is not significantly compromised and, thus, frequently go undetected by the conventional contrast enhanced MRI. This is a major reason for recurrence and relapses because it is difficult to resect completely during surgical procedures. In addition, a contrast-enhanced MRI cannot differentiate recurrent tumors from post-treatment inflammation caused by the radiation or the surgical processes, which poses additional challenges to evaluate of effectiveness of radiation therapy. In this work, we address this critical need for detection of infiltrating glioma with a new approach by using Targeted Quadruple Mutant IL-13 (TQM-13) liposomes encapsulated gadolinium. Method We use interleukin-13 (IL-13) as targeting agent because 75% of glioma cells overexpress IL-13Rα2 significantly1. For this study, we are interrogating the relative efficacy of liposomes conjugated with wild type IL-13 to a variant of IL-13, known as Targeted quadruple mutant13 (TQM-13) that has been shown to be more selective for the IL-13Rα2 and binds with higher affinity than the wide type. As shown in Fig. 1, TQM binds with IL13Rα2, not binds to IL-13Rα1 and IL-4Rα, which makes it a more specific targeting agent for glioma. Test this hypothesis, we conjugated TQM-13 and wild type IL-13 with liposome encapsulated with MRI contrast agent Magnevist that denoted as TQM-13-liposomes-Gd and IL-13-liposomes-Gd, respectively. The intracranial tumor was produced by the injection of U87-luciferase cells into the brain of the athymic nude mice. After intracranial tumor growth was confirmed by IVIS (in vivo imaging system), two groups mouse underwent MRI after injection of TQM-13-liposomes-Gd and Magnevist, or IL-13-liposomes-Gd and magnevist in two consecutive days. The mice were then sacrificed and the brain was collected for histology (H&E) and immunohistochemistry study using antibody of IL-13Rα2 and ionized calcium-binding adapter molecule 1 (Ib-1) (microglia upregulation marker). Results: As shown in Fig. 2, our targeted MRI agent, TQM-13-liposomes-Gd, produced desirable MRI contrast, delineating tumor. The targeted liposomes were able to detect infiltrating tumor that Magnevist cannot 2,3. As shown in Fig. 2B, the inflamed tissues in the boundary region with needle track is enhanced less by TQM-13-liposme than that of Magnevist. The histology and immunohistochemistry in the same area in Fig 3 confirmed tissue classifications and differential contrast enhancements in Fig. 2B. Conclusion: TQM13-liposomes-Gd is more specific for tumor detection than conventional MRI contrast agent such as Magnevist. This novel property is clinically valuable for differentiation of tumor re-occurrence and pseudo progression during therapy.Acknowledgements
No acknowledgement found.References
1. Debinski W, Gibo DM: Molecular expression analysis of restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol Med 6:440-449, 2000.
2. Liu X, Madhankumar AB, Miller PA, Duck KA, Hafenstein S, Rizk E, Slagle-Webb B, Sheehan JM, Connor JR, Yang QX: MRI contrast agent for targeting glioma: interleukin-13 labeled liposome encapsulating gadolinium-DTPA. Neuro Oncol (2016) 18 (5): 691-699 first published online October 31, 2015 doi:10.1093/neuonc/nov263
3. Madhankumar AB, Slagle-Webb B, Mintz A, Sheehan JM, Connor JR: Interleukin-13 receptor-targeted nanovesicles are a potential therapy for glioblastoma multiforme. Mol Cancer Ther 5:3162-3169, 2006.
Figures
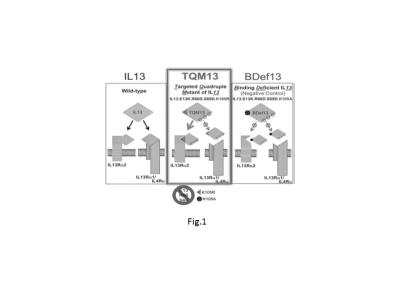
Fig. 1. Schematic of IL-13, TQM13, and BDef13 and their interaction with the physiologically abundant IL-13Ra1 and the tumor-restricted IL-13Rα2. Although IL-13 binds to both receptors, TQM-13 contains hotspot mutations that prevent binding to the physiologically abundant IL-13Rα1/IL4Ra but not the cancer-restricted IL-13Rα2. BDef13 is a negative control that differs from TQM-13 only in the 105th amino acid position which dramatically decreases affinity towards both IL-13 receptors. Nguyen et al. Neuro-Oncology 14(10):1239–1253, 2012.

