3069
A Bright Contrast Labelling Approach for Non-Invasive MR Imaging of Biomaterials1Institute of Biomaterials & Biomedical Engineering, University of Toronto, Toronto, ON, Canada, 2Ted Rogers Centre for Heart Research, Translational Biology & Engineering Program, Toronto, ON, Canada, 3Pharmaceutical Sciences, Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON, Canada, 4Department of Physical and Environmental Sciences, University of Toronto Scarborough, Toronto, ON, Canada, 5Department of Chemistry, University of Toronto, Toronto, ON, Canada, 6Edward S. Rogers Sr. Department of Electrical & Computer Engineering, University of Toronto, Toronto, ON, Canada
Synopsis
Tissue engineered biomaterials have the potential to regenerate almost every tissue type. One difficult aspect to advancing this technology is determining the properties and fate of these materials once introduced in vivo. Non-invasive imaging technology such as MRI holds significant potential for monitoring implanted biomaterials. Few novel approaches to directly image biomaterials have recently been developed; however, most are designed for specific materials or utilize iron oxides with limited specificity. In this study, we investigate a novel approach to labelling biomaterials with a highly efficient T1 agent and a biologically derived adhesive, which allows for accurate and sensitive detection in vivo.
Target Audience
Tissue engineer, regenerative medicinePurpose
Biomaterial grafts are essential ingredients in many tissue engineering strategies, as they allow cells to penetrate, attach, and migrate; they retain biochemical factors conducive to tissue growth; and they biodegrade over time at a rate ideally matched to that of new tissue production. Non-invasive direct imaging of the graft is important for evaluating these properties and their performance in vivo. Few novel approaches to directly image biomaterials have recently been developed; however, most are designed for specific materials or utilize iron oxides with limited specificity1,2,3,4. In this study, we investigate the design and use of a highly efficient T1 contrast agent, MnP-NH2, and a biologically derived adhesive, polydopamine (PDA), for direct imaging of conventional biomaterials.Methods
The MnP-NH2 contrast agent was synthesized and characterized as previously reported5. The adhesive monomer, dopamine hydrochloride (Sigma Aldrich) was polymerized to form PDA in slightly basic media at 4oC, similar to previous reports6. Conventional biomaterials tested include collagen hydrogel (Bovine Type 1 Collagen; Advanced Biomatrix, San Diego, CA) and decellularized bladder tissue, prepared as previously reported7. Biomaterials were labelled in vitro with varying concentrations of MnP-NH2 and PDA and then extensively washed prior to imaging on a 3.0T scanner (Achieva 3.0 T TX, Philips Medical Systems) using a 32-channel head coil. T1 mapping was performed using inversion recovery turbo spin echo: TR=3000 ms, TE=18.453 ms, FOV= 120 X 120 mm, 3 mm slices, 0.5 mm x 0.5 mm in-plane resolution, and T1= [50, 100, 250, 500, 750, 1000, 1250, 1500, 2000 and 2500] ms. Animal studies were performed on five female adult Sprague Dawley rats (Charles River Laboratories) weighing 250-300 g under our institutionally approved protocol. Collagen hydrogels were injected subcutaneously, and animals were imaged using an 8-channel wrist coil on a 3.0T scanner. Sagittal 2D fat-suppressed T1-weighted spin-echo images were acquired with slice thickness of 3 mm and 0.6 mm x 0.6 mm in-plane resolution. Animals were imaged longitudinally up to 22 days and sacrificed afterwards for gross dissection.Results
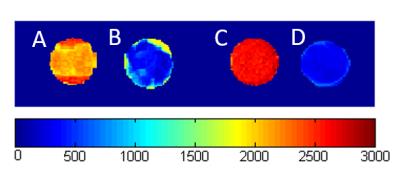
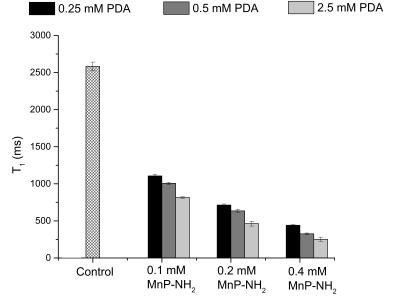
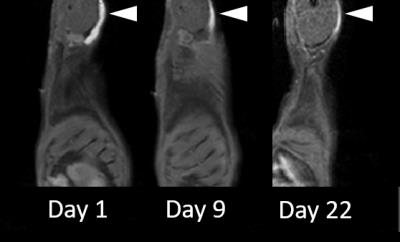
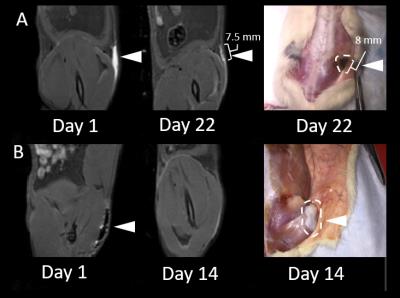
The feasibility of labelling both ECM tissue and collagen hydrogels is shown in Fig. 1; T1-maps show a significant decrease in T1 in labelled biomaterials compared to unlabelled. Unlabelled decellularized bladder and unlabelled collagen hydrogel exhibit high T1 values of 2133.34 + 79.15 ms and 2583.38 + 58.12 ms, respectively; however, the materials labelled with 0.4 mM MnP-NH2 and 5 mM PDA exhibited very low T1 values of 329.56 + 91.15 ms and 289.33 + 28.11 ms, respectively. Fig. 2 compares changes in T1 across different concentrations of MnP-NH2 and PDA in collagen hydrogels. Fig. 3 shows in-vivo fat-saturated T1-weighted spin echo images of an injected labelled collagen hydrogel. Measurements of the contrast-to-noise ratio (CNR) of the labelled grafts over time yielded a consistent decrease, suggestive of bulk degradation, a known property of collagen gels (CNR Values- Day 1: 242, Day 9: 152, Day 22: 107). Fig. 4 shows in-vivo fat-saturated T1-weighted spin echo images and gross dissection of an injected unlabelled and labelled collagen hydrogel. No rats suffered toxic or lethal effects from labelled or unlabelled gels.Discussion
MnP-NH2 and PDA is a highly efficient and sensitive T1 approach for direct labelling of biomaterials. Once optimized a significant reduction in T1 was achieved with concentrations as low as 0.1 mM MnP-NH2 and 0.25 mM PDA. MR labelled collagen gels could be monitored with MR throughout the course of degradation. Correspondence in graft geometry and dimensions between MR and the explant suggest that our approach can be used to accurately monitor biomaterials in vivo. Most importantly our labelling method did not cause toxic or lethal effects.Conclusion
We have presented a promising new method for sensitive and accurate MRI tracking of biomaterials that will significantly improve and accelerate material assessment and development for tissue engineering and regenerative medicine.Acknowledgements
D.A. Szulc is supported by the Ontario Graduate Scholarships. H.-L. M. Cheng is funded by the Heart and Stroke Foundation of Canada, NSERC Discovery Awards, IBBME Director's KickStart Awards, the Ontario Research Fund, and the Canada Foundation for Innovation.References
1. Mertens, M. E. et al. Iron Oxide-Labeled Collagen Scaffolds for Non-Invasive MR Imaging in Tissue Engineering. Advanced Functional Materials 2014, 24 (6), 754-762.
2. Karlfeld-Sulzer, L. S. et al. Protein Polymer MRI Contrast Agents: Longitudinal Analysis of Biomaterials In Vivo. Magentic Resonance in Medicine 2011, 65 (1), 220-228.
3. Dorsey, S. M. et al. Visualization of Injectable Hydrogels Using Chemical Exchange Saturation Transfer MRI. ACS Biomaterials Science & Engineering 2015, 1 (4), 227-237.
4. Mertens, M. E. et al. (2015). USPIO-labeled textile materials for non-invasive MR imaging of tissue-engineered vascular grafts. Biomaterials 39, 155- 163.
5. Jing, L. et al. Covalent attachment of Mn-porphyrin onto doxorubicin-loaded poly(lactic acid) nanoparticles for potential magnetic resonance imaging and pH-sensitive drug delivery. Acta Biomater 2013, 9 (12), 9434-9441.
6. Lee, B. P. et al. Mussel-Inspired Adhesives and Coatings. Annual Review of Materials Research 2011, 41 (1), 99-132.
7. Evren, S. et al. Urinary Bladder Tissue Engineering Using Natural Scaffolds in a Porcine Model: Role of Toll-Like Receptors and Impact of Biomimetic Molecules. Cells Tissues Organs 2010, 192 (4), 250–261.
Figures