3068
In vitro and in vivo detection of treatment-induced apoptosis using ultrasmall superparamagnetic iron oxide (USPIO)-conjugated Annexin V: A pilot study1Clinical Radiology, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan, 2Innovation Center for Medical Redox Navigation, Kyushu University, 3Meito Sangyo Co., LTD, 4Guerbet Japan KK
Synopsis
Apoptosis is involved in many pathological processes. Early detection of treatment-induced apoptosis in malignant tumors is clinically useful for a decision making in therapeutic strategy. Phosphatidylserine (PS) expressed on the cell membrane is known to be a marker of apoptosis which can be detected by probes with Annexin V. In this study, we newly synthesized USPIO-conjugated Annexin V and investigated its potential in the use in apoptosis imaging in both in vitro and in vivo studies.
Purpose
Dysregulation of apoptosis is associated with many pathologic processes such as cancers, autoimmune and neurodegenerative disorders. It is of great clinical value to detect apoptotic changes. Especially, the early detection of therapeutic effect on malignant tumors is helpful for a decision making in a treatment strategy. Phosphatidylserine (PS) expressed on the cell membrane is known to be a marker of apoptosis. Annexin V can be a sensitive tool in the detection of apoptotic cells since this has a high affinity for PS. So far, researchers have investigated the detection of diseased tissue with, for example, radioactively labeled Annexin V1-4. USPIO nanoparticles have been used as contrast agents for molecular MR imaging. Generally, USPIO-based contrast agents have higher T1- and T2- relaxivity than Gd-based agents. In this study, we newly synthesized USPIO-conjugated Annexin V (USPIO-Annexin V) and investigated its potential in apoptosis imaging in both in vitro and in vivo studies.Methods
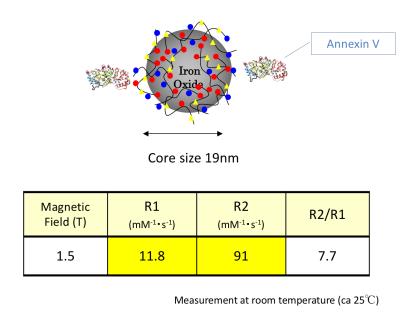
Contrast agent: Annexin V binding carboxymethyl diethylaminoethyl dextran magnetite (CMEADM) USPIO particles (Meito Sangyo Co. Ltd., Aichi, Japan) were newly synthesized for this study. The core size was 19nm and 1-2 Annexin V bond per particle. The R1 and R2 relaxivity at 1.5 T was 11.8/mM/sec and 91/mM/sec, respectively(Fig. 1).
In vitro binding: Jurkat cells were incubated for 6 hours to induce apoptosis by 15μM camptothecin. Apoptotic cells (5×107) were incubated with USPIOs at a concentration of 0.089 mmol Fe/L for 60 minutes. After incubation, free USPIOs were removed by centrifuge and USPIO binding apoptotic cells were extracted. T2 values of cell suspension were measured by 0.47T NMR spectrometer and iron concentration was then calculated.
Animal Model: EL-4 cells (2×106) were injected subcutaneously in the flanks of C57BL/6 mice (Charles river laboratories Japan, Inc., Kanagawa, Japan). At two weeks after the implantation, the animals were treated with an intraperitoneal injection of etoposide (67 mg/kg) (SANDOZ, Yamagata, Japan) and cyclophosphamide monohydrate (100 mg/kg) (Shionogi & Co., Ltd., Osaka Japan)5.
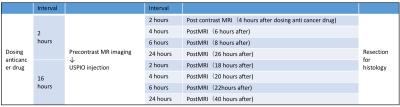
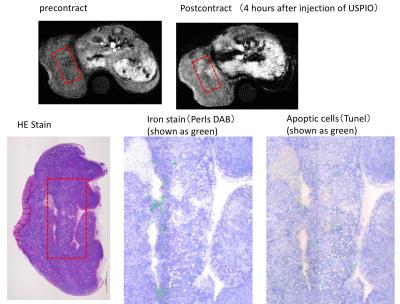
MRI: MRI were conducted on a 1.5T animal imager (MR VivoL VA, DS Pharma Biomedical, Osaka, Japan). Following a pre-contrast imaging, post-contrast images were obtained at 2, 4, 6, and 24 hours after the intravenous injection of USPIO (100μmol Fe/kg). The interval between the administrations of anti-tumor drug and contract agent was 2 or 16 hours (Fig. 2). T1-weighted images were obtained in the following parameters; 2D-gradient-echo; TR/TE=130/2.5msec; FA90; FOV=35mm; matrix=128*128; thickness=1mm; 5 slices; average=10; acquisition time=2min44sec. Signal intensities (SIs) were measured in tumors and SI contrast ratios (Pre/Post) were calculated. USPIO without Annexin V was also used for a reference. Histological evaluations: Tissues were stained with HE, TUNEL and Perls DAB.
Results and Discussion
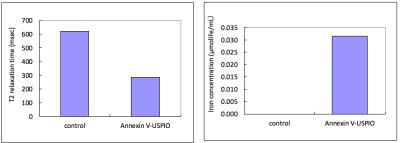
In vitro binding: Apoptotic cells incubated with USPIO-Annexin V had shortened T2 values, suggesting their bindings (Fig. 3). It was estimated that total amount of iron which bonded to apoptotic cells were 0.031μ mol Fe/mL and approximately 35% of USPIO-Annexin V bonded with apoptotic cells. This result showed the potential of binding of USPIO-Annexin to PS in apoptotic cells.
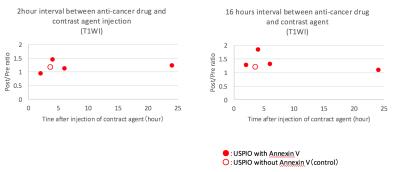
MRI: SI contrast ratios (Post/Pre) on T1-weighted images were increased (1.46 or 1.86) at 4 hours after the injection of USPIO-Annexin V (Fig. 4). This indicates the in-vivo detection of apoptosis induced by anti-cancer drugs. In this study, USIPO was used as a T1-shortening agent since this has high T1-relaxivity. Previous studies also used USPIO for T1-weighted imaging in MR angiography and brain tumor imaging6-9. On the other hand, the SI ratio on T2-weighted images were unchanged at any time points (not shown). The imaging parameters need to be optimized for T2-weighted imaging. EL-4 is a well-known model of apoptotic cell. Changes in the cell membrane include a translocation of PS from the inner to outer leaflets of membran10. A previous in vitro study using EL-4 showed that Annexin V positive cells rapidly increased by 20% at 1 hour after a stimulation and reached 80% at 6 hours; however, DNA fragmentation lagged behind the translocation of PS11. Thus, in this study, we set the time interval to include the initiation and end of apoptosis. The SI of tumor was heterogeneously increased at 4 hours after the injection of USPIO-Annexin V (Fig. 5). The histology showed that the area with increased SI corresponded to the area with iron and apoptotic cells, which proved the in vivo binding.
Conclusion
This pilot study suggests that newly developed USPIO-Annexin V has a potential for early detection of the chemotherapeutic effect by MRI although the experimental system should be optimized.Acknowledgements
No acknowledgements found.References
1. Am J Nucl Med Imaging 2015:5:27-37
2. J Nucl Med 2005:46:2035-2050
3. J Nucl Med 2011:52:1786-1794
4. Molecules 2015:20:4902-4914
5. Radiology 2008:246:854-862
6. AJNR Am J Neuroradiol 2002:23:510-519
7. Radiology 2004:231:474-481
8. Brain 2008:131:800-807
9. Jpn J Radiol 2012:30:832-839
10. Bioorg Med Chem 2005:13: 5035–5042
11. J Cell Mol Med 2002:6:82-92
Figures