3066
Highly Sensitive Magnetic Nanoprobe for Detection of ErbB2-expressing Cancer1Department of Radiology, Yonsei University College of Medicine, Seoul, Korea, Republic of, 2Nanomedical National Core Research Center, Yonsei University, Seoul, Korea, Republic of
Synopsis
ErbB2, which belongs to the epidermal growth factor receptor (EGFR) family, plays a key role in human malignancies. ErbB2 is overexpressed in approximately 30% of human breast cancers and in many other cancer types, including stomach, bladder, ovarian and lung carcinomas. The objective of this study is the development of anti-ErbB2 aptamer-modified T2 contrast agent based on magnetic nanoprobe (AptErbB2-MNP) having high-specificity onto Erbb2-expressing cancer. For confirmation of AptErbB2-MNP as T2 contrast agent, T2 relaxivity and hydrodynamic diameter was measured. in vitro binding affinity tests were conducted not only recombinant ErbB2 proteins but also the live cells. in vivo targeting ability was verified by in vivo MRI analysis.
Introduction
Erbb2, which belongs to the epidermal growth factor receptor (EGFR) family, plays a key role in human malignancies. Erbb2 is overexpressed in approximately 30% of human breast cancers1 and in many other cancer types, including stomach, bladder, ovarian and lung carcinomas.1-4 For this reason, Erbb2 can be selected as important biomarker in radiological diagnosis of cancer. The objective of this study is the development of anti-ErbB2 aptamer-modified T2 contrast agent based on magnetic nanoprobe (AptErbB2-MNP) having high-specificity onto Erbb2-expressing cancer cells.Materials and Methods
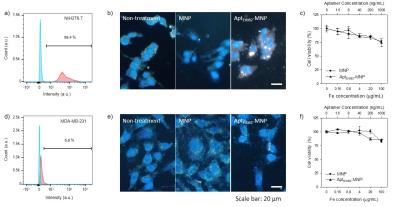
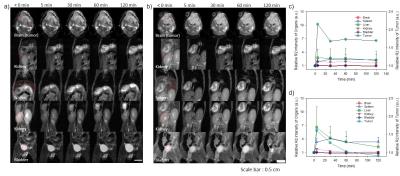
AptErbB2-MNP was prepared by conjugation with 5’-thiol modified anti-ErbB2 aptamer (AptErbB2-SH) and maleimidylaed magnetic nanoparticle.5 For the characterization as MRI contrast agent of AptErbB2-MNP, the T2 relaxivity and the hydrodynamic diameter were measured (control group: non-labeled magnetic nanoparticle, MNP). AptErbB2-MNP was assayed for its binding affinity onto recombinant ErbB2 proteins compared with AptErbB2-SH. In this assay, all aptamers were labeled by [α-32P]ATP on 3’-end of aptamers.6 The in vitro Erbb2-targeting efficacy of AptErbB2-MNP was investigated by dark field-fluorescence microscopy in NIH3T6.7 (Erbb2+), and MDA-MB-231 (Erbb2-) cell lines. The in vitro cell viability assay was also performed 24 hour after treatment of AptErbB2-MNP. For the in vivo MR imaging, xenograft mice model was prepared by the injection of NIH3T6.7 cells (1.0 × 106 cells/mouse) into brain with frontal lobe coordinates 2 mm lateral and 1 mm anterior to the bregma of BALB/c nude mice. After 10 days, the tumor-targeted MR imaging was conducted by intravenous injection of AptErbB2-MNP onto tumor-formed mice at 3T clinical MRI instrument using human wrist 8ch-array coil.Results and Discussion
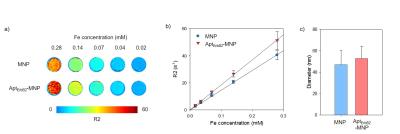
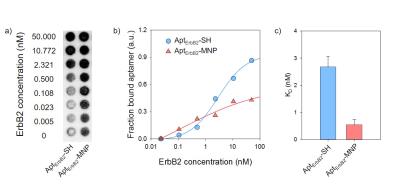
The R2 relaxivity of MNP and AptErbB2-MNP was measured as 145.73 and 184.97 mM-1s-1 respectively. The hydrodynamic diameter was 47.05 $$$\pm$$$ 13.19 and 52.79 $$$\pm$$$ 11.32 nm. This values is similar with Feridex I.V.TM (Bayer HealthCare Pharmaceuticals Inc. NJ, USA). The in vitro binding affinity (KD) of AptErbB2-SH was 2.67 $$$\pm$$$ 0.40 mM, but AptErbB2-MNP was 0.54 $$$\pm$$$ 0.20 mM. The naked ErbB2 aptamer (AptErbB2-OH) has a KD of under 0.78 mM, the increase of AptErbB2-SH’ KD seems to be caused by presence of the thiol groups. This assumption has some credibility because the KD AptErbB2-SH was recovered after became AptErbB2-MNS prepared by conjugation between AptErbB2-SH and maleimidylaed MNP. The results of in vitro targeting analysis were shown that AptErbB2-MNP has the specificity onto NIH3T6.7 cells (fig. 3). In dark field-fluorescence microscopy analysis, AptErbB2-MNP was more attached to NIH3T6.7 cells than MDA-MB-231 cells. In case of MNP-treatment experiments, there is very little difference in attaching tendency both NIH3T6.7 and MDA-MB-231 cells. In both cell lines, the cell viability was showed over 75%, the notable inhibition of cell viability wasn’t shown. The targeting ability of AptErbB2-MNP was also verified in vivo MR imaging. The T2 weighted imaging was conducted until 120 min since the injection of AptErbB2-MNP (fig. 4). The relative R2 intensity graph was obtained from the red colored ROIs in T2 weighted images. The ROIs was drawn at the sites which were displayed the large contrast increase between pre- (< 0min) and post-injection (5 min) of MNP or AptErbB2-MNP. The up to 220%-increased signal intensity appeared at 5 min on T2 weighted images of AptErbB2-MNP-injected mice and then the decreased intensity was maintained over 170%. In case of MNP, the up to 160% increase of signal intensity was showed at 5 min, however it was gradually decreased and finally, the signal intensity returned to base line level. Both of them were not shown the contrast enhancement effect at the bladder, and normal region of brain, but MNP was displayed that the accumulated amount of approximately 200% more than AptErbB2-MNP in liver and spleen which are the main organs of reticuloendothelial system (RES). These phenomena suggested that AptErbB2-MNP has the specificity for ErbB2-expressing cancer and higher avoiding ability against RES than non-specific contrast agent.Conclusion
The possibility of AptErbB2-MNP as ErbB2-specific T2 contrast agent was confirmed by in vitro and in vivo analysis. Patient with Erbb2-overexpressing breast cancer is tended to have substantially lower survival rate than patient whose cancer dose not overexpressing Erbb2.8 In addition, Erbb2-overexpressing lead to increased breast cancer metastasis.9-11 Therefore, Erbb2-target MRI contrast agent might be useful to diagnose the patient has Erbb2-overexpressing breast cancer. In conclusion, we expect that the result of this work will be a promising strategy for diagnosis of Erbb2-overexpressing cancer and treatment for patients.Acknowledgements
This work was supported by a National Research Foundation (NRF) grant funded by the Korea government, Ministry of Education and Science Technology (MEST, 2015R1A2A1A05001887) and also supported by a faculty research grant of Yonsei University College of Medicine for (6-2014-0032).References
1. Slamon DJ, Godolphin W, Jones LA. et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science.1989;244(4905):707–712.
2. Lemoine NR, Jain S, Silvestre F. et al. Amplification and overexpression of the EGF receptor and c-erbB-2 proto-oncogenes in human stomach cancer. Br J Cancer. 1991;64(1):79–83
3. Sauter G, Moch H, Moore D. et al. Heterogeneity of erbB-2 gene amplification in bladder cancer. Cancer Res. 1993;53(10):2199–2203
4. Tateishi M, Ishida T, Mitsudomi T. et al. Prognostic value of c-erbB-2 protein expression in human lung adenocarcinoma and squamous cell carcinoma, Eur J Cancer. 1991;27(11):1372–1375
5. Dan Heo et al. Maleimidyl magnetic nanoplatform for facile molecular MRI, Nanotechnology 2014;25:275102
6. Myoung-Eun Han et al. Development of an aptamer-conjugated fluorescent nanoprobe for MMP2. Nanoscale Res Lett. 2014; 9(1): 104.
7. Weinstein, J. S. et al. Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J. Cereb. Blood Flow Metab. 2010;30(1):15-35
8. Dogan L et al. Prognosis in hormon receptor negative breast cancer patients according to ERBB2 status. Neoplasma 2008;55(6):544–548
9. Tan M, Yao J, Yu D. Overexpression of the c-erbB-2 gene enhanced intrinsic metastatic potential in human breast cancer cells without increasing their transformation abilities. Cancer Res. 1997;57:1199–1205
10. Moody SE, Sarkisian CJ, Hahn KT. et al. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell. 2002;2(6):451–461
11.
Holbro T, Civenni G, Hynes NE. The ErbB
receptors and their role in cancer progression. Exp Cell Res.
2003;284(1):99–110
Figures