3064
A novel tracer for non-invasive atherosclerotic plaque phenotyping by PET/MR imaging1Translational and Molecular Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Medical Biochemistry, Academic Medical Center, Amsterdam, Netherlands, 3Division of Cardiovascular Diseases, Sulpizio Cardiovascular Center, Department of Medicine, University of California, La Jolla, San Diego, CA, USA., 4Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, USA
Synopsis
Atherosclerotic plaques that rupture can cause stroke or myocardial infarction. Oxidation specific epitopes (OSE), as present in oxidized LDL (OxLDL), are hallmarks of vulnerable plaques . We have developed the PET radiotracer 89Zr-LA25 that targets OSE. Integration of 89Zr-LA25 PET imaging with previous validated techniques enables ‘’vulnerable’’ plaque phenotyping by PET/MRI
Introduction
Atherosclerosis is the major underlying cause of cardiovascular disease. Oxidation specific epitopes (OSE) are well defined danger-associated molecular patterns that activate inflammatory pathways leading to initiation and progression of atherosclerotic plaques.1 To detect (sub)clinical atherosclerosis, a variety of imaging techniques are available. However, current clinical techniques do not characterize the atherosclerotic plaque well, but merely detect the level of stenosis. In the past decade, molecular imaging has provided new insights in pathophysiology at a cellular and molecular level. It therefore allows phenotyping and identifying features of plaque progression that precede a possible plaque rupture.2
We have developed a new OSE-specific PET imaging probe and evaluated this tracer using a multimodality imaging approach.
Methods
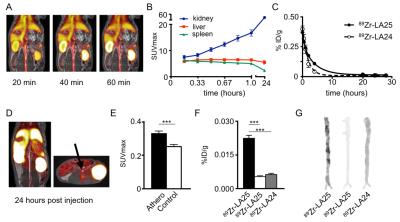
Human monoclonal antibody Fab LA25 was generated from a phage display library screened for OSE binding. LA25 was modified with the chelator deferoxamine B and radiolabeled with Zirconium-89 (89Zr-LA25). Pharmacokinetics, biodistribution and plaque specificity were extensively assessed in cholesterol-fed apoE-/-mice and a rabbit model of atherosclerosis. For in vivo imaging, a clinical positron emission tomography with magnetic resonance imaging (PET/MRI) system was used. First, the level of vessel wall inflammation was determined by a 18F-FDG PET/MR scan. The next day, after intravenous injection with 89Zr-LA25 or 89Zr-LA24, a non-specific Fab, rabbits were scanned in a dynamic fashion for 1 hour (fig.1A) with simultaneous acquisition of dynamic contrast enhanced MRI (DCE-MRI) to evaluate neovascularization. An additional scan was performed 24 hours post injection, after which the animals were sacrificed to assess macrophage content by ex vivo near infrared fluorescence (NIRF).Results
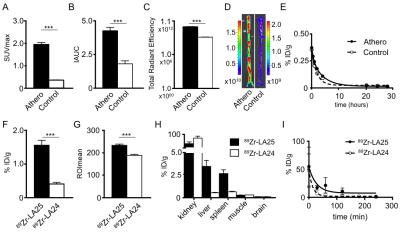
Dynamic PET imaging data revealed an increase in renal signal over time (fig.1B) indicative of renal clearance. The weighted half-life in atherosclerotic rabbits was 2.14h for 89Zr-LA25, 1.20h for 89Zr-LA24 (fig.1C and fig. 2E) and 1.31h for 89Zr-LA25 in healthy control rabbits. After 24 hours, PET/MR imaging (fig.1D) showed a significant higher uptake of 89Zr-LA25 in the abdominal aorta of atherosclerotic rabbits compared with control (P=0.0003, fig.1E), confirmed by gamma counting (P<0.0001, fig.1F) and autoradiography (fig.1G). 18F-FDG PET/MR imaging, DCE-MRI and NIRF showed significant higher uptake in atherosclerotic rabbits (Fig. 2A-D). Similar results were obtained in mice (Fig. 2F-I). Uptake of 89Zr-LA24, was significantly lower than that of 89Zr-LA25 (P<0.0001 fig.1F, G).Conclusion
89Zr-LA25, a novel radiotracer targeting OSE, enables phenotyping of atherosclerosis using PET/MRI. In combination with previously validated techniques we are now able to visualize and quantify inflammation (18F-FDG), neovascularization (DCE-MRI) and OSE, which are key features of a vulnerable plaque. Moreover, this radiotracer could further help characterize the process of atherosclerosis and ultimately serve as a biomarker in a clinical setting to evaluate therapeutic interventions.Acknowledgements
No acknowledgement found.References
1. Briley-Saebo KC, Nguyen TH, et al. In vivo detection of oxidation-specific epitopes in atherosclerotic lesions using biocompatible manganese molecular magnetic imaging probes. J Am Coll Cardiol. 2012;59(6):616-626.
2. Mulder WJM, Jaffer F A, et al. Imaging and Nanomedicine in Inflammatory Atherosclerosis. Sci Transl Med. 2014;6:239sr1.
Figures