3063
Simultaneous imaging of drug delivery with SPIO-based MRI and drug therapy with pHe readout from BIRDS1Biomedical Engineering, Yale University, New Haven, CT, United States, 2Department of Radiology & Biomedical Imaging, Yale University, New Haven, CT, United States
Synopsis
Since acidification of the extracellular environment is a hallmark of cancer pathogenesis, successful therapy may manifest as normalization of pHe. We have shown that quantitative pHe measurement is possible with BIRDS is possible even in the presence of superparamagnetic iron oxide nanoparticles (SPIO-NPs). Because SPIO-NPs have been used to image and track drug delivery, we envisage co-injection of BIRDS agents and NPs, containing drugs and SPIO, as a new protocol that can track drug delivery to tumors, concurrently map tumor location and size (by MRI), and at the same time measure therapeutic efficacy through changes in tumor pH (by BIRDS).
Objectives
Brain’s microvasculature is disrupted in several pathologies, including cancer. Breakthroughs in glioma imaging and therapy exploit the fact that nanoparticles enter the interstitial space by extravasation. Thus co-injection of tumor-targeted nanoparticles containing drugs (D-NPs) and superparamagnetic iron oxide nanoparticles (SPIO-NPs) can enable safe delivery of chemotherapy to the tumor (via D-NPs) and imaging of drug biodistribution (via SPIO-NPs) (1-4). However, molecular readout beyond the generated contrast is inhibited by the large magnetic field gradients created by SPIO-NPs. Because lower extracellular pH (pHe) is a hallmark of cancer pathogenesis that promotes invasion and resistance to therapy, a particular need exists for non-invasive methods to report on tumor pHe quantitatively. We have previously demonstrated quantitative pHe measurement with biosensor imaging of redundant deviation in shifts (BIRDS), which utilizes paramagnetically-shifted resonances of non-exchangeable protons on shift agents (e.g., TmDOTP5-)(5-8).Methods
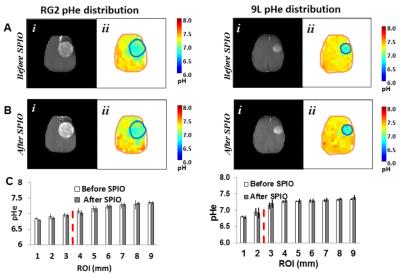
Male Fischer 344 rats (200-250g) were inoculated with 9L and RG2 tumors. We measured brain pHe in 9L and RG2 glioma-bearing rats by BIRDS at 9.4 T using TmDOTP5- before and after infusion of SPIO (14 mg/kg). All animals received a 1 mmol/kg dose of TmDOTP5- by IV injection. Renal ligation or Probenecid infusion (100mg/kg) was used to inhibit renal clearance of TmDOTP5-(9). Since proton relaxation is significantly enhanced by pseudo-contact interactions with unpaired electrons of Tm3+, we hypothesized that BIRDS-based pHe readout from TmDOTP5- remains uncompromised by the presence of SPIO-NPs.Results
Superb MRI contrast was achieved upon infusion of SPIO. The tranverse relaxation, (R2) enhancement was region dependent; highest within the tumor and was lowest in healthy brain farthest from the center of mass of tumor. While superior MRI contrast of the tumor core was revealed upon SPIO-NPs in 9L and RG2 gliomas, the average pHe measured in absence and presence SPIO-NPs were very similar, with pHe values of 6.9 inside the tumor core and pHe values of 7.3 beyond the tumor boundary. Brain pHe values were lowest in the tumor core and increased regionally as a function of the distance from the center of mass of the tumor. Furthermore, we found that in less aggressive gliosarcomas like 9L, the acidification of the extracellular pHe was restricted to the tumor boundary (pH 6.88 +/- 0.08 inside the tumor and 7.29 +/- 0.05 outside the tumor). For the aggressive gliomas like RG2 the acidification of the extracellular environment occurred both within and beyond the tumor boundary (pH 6.98+/- 0.14 in the tumor core, pH 7.08 +/- 0.09 at the tumor edge and pH 7.26 +/- 0.09 farther from the tumor). These pHe patterns of different tumor types were unchanged by presence of SPIO-NPs. The average pHe after SPIO infusion was pH 6.88 +/- 0.08 inside the tumor and 7.29 +/- 0.05 outside the tumor for the 9L tumor. For RG2 tumors, the average pHe after SPIO infusion was 6.98+/- 0.14 in the tumor core, pH 7.08 +/- 0.09 at the tumor edge and pH 7.26 +/- 0.09 farther from the tumor). Additionally, the patterns of pHe distribution and relaxation enhancement were independent of the method used to inhibit renal clearance.Conclusions
We envisage co-injection of TmDOTP5- and nanoparticles, containing drugs and SPIO, as a new protocol that can track delivery of therapeutic agents to tumors, concurrently map tumor location and size (by MRI), and at the same time measure changes in tumor pHe in response to therapy (by BIRDS).Acknowledgements
Supported by NIH grants:
RO1 NCI
RO1 CA.140102
P30 NS-052519
Special thanks to all MRRC colleagues at the Yale school of medicine
References
1. G. Strohbehn, D. Coman, L. Han, R. R. Ragheb, T. M. Fahmy, A. J. Huttner, F. Hyder, J. M. Piepmeier, W. M. Saltzman, and J. Zhou, J Neurooncol 121, 441 (2015).
2. S. Geninatti Crich, M. Cadenazzi, S. Lanzardo, L. Conti, R. Ruiu, D. Alberti, F. Cavallo, J. C. Cutrin, and S. Aime, Nanoscale (2015).
3. Z. Zhen, W. Tang, H. Chen, X. Lin, T. Todd, G. Wang, T. Cowger, X. Chen, and J. Xie, ACS Nano 7, 4830 (2013).
4. J. Zhou, T. R. Patel, R. W. Sirianni, G. Strohbehn, M. Q. Zheng, N. Duong, T. Schafbauer, A. J. Huttner, Y. Huang, R. E. Carson, Y. Zhang, D. J. Sullivan, Jr., J. M. Piepmeier, and W. M. Saltzman, Proc Natl Acad Sci U S A 110, 11751 (2013).
5. C. Baudelet and B. Gallez, Magn Reson Med 48, 980 (2002).
6. D. Coman, Y. Huang, J. U. Rao, H. M. De Feyter, D. L. Rothman, C. Juchem, and F. Hyder, NMR Biomed (2016).
7. D. Coman, H. K. Trubel, and F. Hyder, NMR Biomed 23, 277 (2010).
8. S. Maritim, Y. Huang, D. Coman, and F. Hyder, J Biol Inorg Chem (2014).
9. Y. Huang, D. Coman, P. Herman, J. U. Rao, S. Maritim, and F. Hyder, NMR Biomed (2016).
10. C. H. Fan, C. Y. Ting, H. J. Lin, C. H. Wang, H. L. Liu, T. C. Yen, and C. K. Yeh, Biomaterials 34, 3706 (2013).
11. R. A. Cardone, V. Casavola, and S. J. Reshkin, Nat Rev Cancer 5, 786 (2005).
12. R. A. Gatenby, E. T. Gawlinski, A. F. Gmitro, B. Kaylor, and R. J. Gillies, Cancer Res 66, 5216 (2006).
13. A. I. Hashim, X. Zhang, J. W. Wojtkowiak, G. V. Martinez, and R. J. Gillies, NMR Biomed 24, 582 (2011).
14. R. Martinez-Zaguilan, E. A. Seftor, R. E. Seftor, Y. W. Chu, R. J. Gillies, and M. J. Hendrix, Clin Exp Metastasis 14, 176 (1996).
15. R. A. Gatenby and R. J. Gillies, Nat Rev Cancer 4, 891 (2004).
Figures