3060
Synthesis of hepatocyte-specific manganese complex with high kinetic stability and MR contrast characteristic for liver cancer imagingHeekyung Kim1, Garam Choi2, Md. Kamrul Islam2, Soyeon Kim2, Ah Rum Baek2, Bo Kyung Sung2, Eunyoung Jeon3, Tae-Jeong Kim3, and Yongmin Chang1,2,4
1BK21 Plus KNU Biomedical Convergence Program, Kyungpook National University, Daegu, Korea, Republic of, 2Medical & Biological Engineering, Kyungpook National University, Daegu, Korea, Republic of, 3Institute of Biomedical Engineering Research, Kyungpook National University, 4Radiology, Kyungpook National University
Synopsis
Novel manganese (II) complex based on EDTA coordination cage bearing benzothiazole aniline (BTA) moiety with high chelation stability was designed and synthesized for use as a liver-specific MRI contrast agent. In addition to forming a hydrophilic, this new hepatobiliary Mn(II) chelate is rapidly taken up by hepatocyte of liver. The magnetic and kinetic properties of Mn(II) complex are higher than commercially available analogue, Mn-DPDP, which was clinically approved MR liver contrast agent. The complex, Mn-EDTA-BTA, was evaluated via in vivo MR imaging to prove high tumor detection sensitivity using animal liver tumor model.
Introduction
The contrast agents (CAs) which make better contrast in MRI are built on the basis of a paramagnetic metal ion. Most clinical contrast agents are based on Gd(III) complexes with seven unpaired electrons, however, it has recently been linked with a medical condition known as nephrogenic systemic fibrosis (NSF). With the increasing safety concerns associated with these potential toxicities of Gd(III) retention in human body, Mn(II) has been attracted as an alternative approaches based on sufficient positive contrast enhancement. In this study, novel manganese (II) complex based on EDTA coordination cage bearing benzothiazole aniline (BTA) moiety (Mn-EDTA-BTA) is presented as a new hepatobiliary MRI contrast agent with high kinetic stability. This new family of contrast agent would be most applicable for liver imaging, especially in liver cancer imaging.Material and methods
All other commercial reagents were purchased from Aldrich or TCI, and used without additional purification. The characterization for Mn(II)-complex in each step was confirmed by microanalysis and spectroscopic techniques such as 1H NMR and HR-FAB-MS. T1 relaxation times were determined using an inversion recovery method with 35 different inversion times (TI) at 1.5 T (64MHz). T2 relaxation times were measured using CPMG (Carr-Purcell-Meiboon-Grill) pulse sequence for multiple spin-echo measurements with 32 different echo times (TE). For in vivo MR study, a tail vein of mice was used for injection (0.05 mmol Mn/kg for Mn-EDTA-BTA and 0.01 mmol Mn/kg for Mn-DPDP) and being performed at 1.5 T MR unit (GE Healthcare, Milwaukee, WI, USA) with a homemade small animal RF coil. Six-week male ICR mouse and HepG2 xenograft mouse were introduced for this study. The mice were anesthetized by 1.5% isoflurane in oxygen. The imaging parameters for spin echo (SE) are as follows; repetition time (TR) = 300 ms; echo time (TE) = 12 ms; 11 mm field of view (FOV); 192 x 128 matrix size; 1.2 mm slice thickness; number of acquisition (NEX) = 8. Transmetallation kinetic study was carried out on 3 Tesla (T) whole body system (Discovery MR750w 3.0T, GE healthcare) at room temperature. 20μL of a 10mM buffered solution (50mM MES buffer, pH 6.0) of ZnCl2 is added to 1mL of a buffered solution of 1mM manganese complex. Transverse (r2) relaxivity was measured as a function of time and the kinetic inertness was evaluated by transverse (r2) relaxivity change (Δr2(t) = r2(0) – r2(t)).Result and discussion
Scheme shows the synthesis of Mn-EDTA-BTA. The r1 relaxivity of Mn-EDTA-BTA is 3.47±0.12 mM−1sec−1, higher than that of Mn-DPDP and Gd-DTPA (2.8 and 3.3 mM−1sec−1, respectively). Figure 1 represents the kinetic stability results of Mn-EDTA-BTA in comparison with commercially available analogues such as MnDPDP and Gd-DTPA. Compared to Mn-DPDP, Mn-EDTA-BTA is sufficiently inert to Mn2+ transmetalation. Furthermore, the pattern of Δr2(t) for Mn-EDTA-BTA is almost same as that for Gd-DTPA revealing that Mn-EDTA-BTA is as stable as Gd-DTPA. Figure 2 shows in vivo MR images of mice before and after bolus injection of Mn-EDTA-BTA (Figure 2a and 2b) and Mn-DPDP (Figure 2c). Figure 2b reveals strong contrast enhancement in liver, kidney after injection followed by gallbladder and intestine. Therefore, these MR images suggest that Mn-EDTA-BTA has dual pathway for excretion including via hepatobiliary and renal pathway. Interestingly, this dual excretion characteristic of Mn-EDTA-BTA is very similar with that of clinically approved gadolinium liver-specific agent, Primovist (Gd-DTPA-EOB). Figure 3 shows in vivo MR images of HepG2 xenograft mice after bolus injection of Mn-EDTA-BTA (Figure 3a) and Mn-DPDP (Figure 3b). Mn-EDTA-BTA shows strong signal enhancement in normal tissue but not in tumor tissue whereas Mn-DPDP shows positive signal enhancement both in normal and tumor tissue suggesting that MnDPDP is taken up by normal hepatocytes and tumor cells. Therefore, Mn-EDTA-BTA showed much higher CNR between tumor and normal liver tissue compared to MnDPDP suggesting significant improvement in tumor detection and characterization. Accordingly Mn-EDTA-BTA shows a good potential as diagnostic agent for liver cancer.Acknowledgements
No acknowledgement found.References
No reference found.Figures
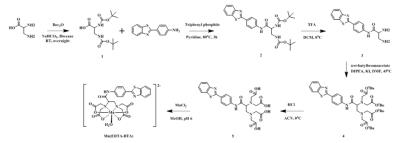
Scheme. Synthesis of
Mn-EDTA-BTA

Figure 1. Transmetalation of 1 mM Mn-DPDP (open circles), Gd-DTPA (open
triangles) or Mn-EDTA-BTA (filled diamonds) by 10 mM Zn2+ at 3T and
293 K
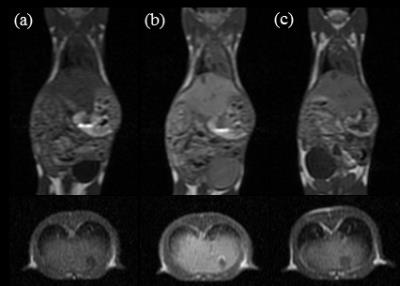
Figure 2. T1-weighted MR images of 6-week male ICR mice: (a)
before injection; 30 min after injection with (b) Mn-EDTA-BTA at a dosage of 0.05
mmol Mn/kg and (c) Mn-DPDP at a dosage of 0.01 mmol Mn/kg
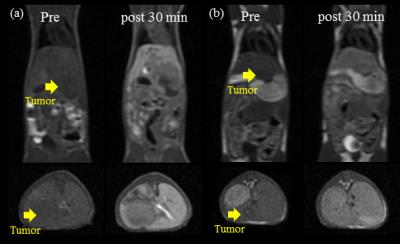
Figure
3. T1-weighted
MR images of HepG2 xenograft mice: (a) before injection, 30 min after injection
with Mn-EDTA-BTA (0.05 mmol Mn/kg) and (b) before injection, 30 min after
injection with Mn-DPDP (0.01 mmol Mn/kg).