3059
Synthesis of Amplifiable Probe Gd-5-HT-DOTAGA and Application to Molecular Imaging of Pulmonary Inflammation1A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2University of Massachusetts Medical School, Worcester, MA, United States, 3Center for Immunology and Inflammatory Diseases, Massachusetts General Hospital, Boston, MA, United States
Synopsis
We report the synthesis of a new amplifiable MR probe that combines the stability of the macrocyclic Gd-DOTA core with the myeloperoxidase-reactive 5-hydroxytryptamide (5-HT) moiety. The relaxivity of Gd-5-HT-DOTAGA is increased 60% in the presence of myeloperoxidase activity. In a mouse model of bleomycin induced lung injury, the change in lung-to-muscle contrast to noise ratio is increased 50% compared to naïve animals, consistent with 3-fold higher Gd lung concentrations measured ex vivo.
INTRODUCTION
5-hydroxytryptamide (5-HT) based probes have been reported previously
for targeting peroxidase activity in tissues associated with inflammation.1,2
Earlier versions of these probes had used bis-5-HT acyclic DTPA-amide based
chelates, which have limited solubility in water. For clinical translation a
more stable chelate with better solubility is needed and here we conjugated
5-HT to the Gd-DOTA core since this chelate is used in the most stable gadolinium
contrast agent used in vivo. We
characterized the complex, its reactivity with myeloperoxidase (MPO) and its
ability to depict the inflammatory component of bleomycin lung injury in vivo.METHODS
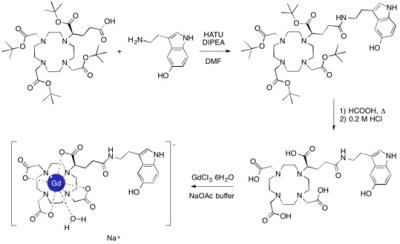
Gd-5-HT-DOTAGA was synthesized in three steps, starting with
the coupling of tert-butyl protected DOTAGA
with serotonin hydrochloride (by using the HATU coupling reagent and DIPEA as a
base), followed by the acidic deprotection (first with formic acid and then
with hydrochloric acid) of the protecting groups and final complexation with
gadolinium chloride in a sodium acetate buffer at pH ~ 6. Relaxivity was
determined by T1 measurement at 0.47 T and 37 °C on pure Gd-5-HT-DOTAGA samples and on samples that were
incubated during 18 h at 37 °C
in presence of MPO and H2O2. Intratracheal administration
of bleomycin (1.0 U/kg) in C57Bl/6 mice was used to induce inflammation and
fibrosis and imaged 20 days post insult. Naïve (n=4) or bleomycin treated (n=4)
mice were imaged with a T1-weighted UTE sequence at 4.7 T before and 1 hour
after intravenous injection of 0.3 mmol/kg Gd-5-HT-DOTAGA. Mice were euthanized
at 2 h after probe injection and Gd biodistribution was performed.
Lung-to-muscle contrast to noise ratios (CNR) were calculated from ROIs in
three slices taken from the base, midpoint, and apex of the lungs. RESULTS
We synthesized Gd-5-HT-DOTAGA at gram scale taking as starting
point (R)-tert-Bu4-DOTAGA3 and serotonin
hydrochloride with a 63 % isolated yield after 3 steps and purifications
(Figure 1). Intermediates were characterized by 1H and 13C
NMR, HPLC, and mass spectrometry (MS), and the final complex by HPLC, MS, and
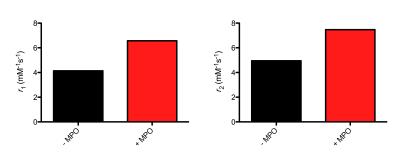
elemental analysis. Incubation of the complex in presence of MPO and H2O2
resulted in a 60% increase in T1 relaxivity and a 50% increase in T2 relaxivity
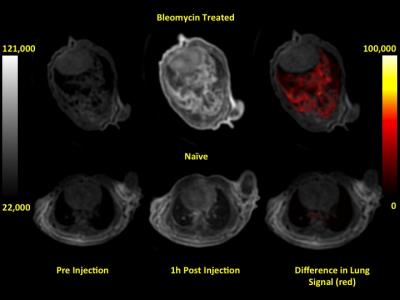
(Figure 2). T1-weighted UTE MR images showed a significant enhancement in the
signal inside the lungs of the bleomycin treated animals (Figure 3). CNR was
calculated before and 1 h after probe administration and ΔCNR was
significantly higher in the bleomycin treated mice compared to naïve mice, ΔCNR = 9.7
± 0.8 vs 6.3 ± 0.8, P = 0.019, respectively. Ex
vivo lung Gd levels were 3 times higher in the bleomycin treated mice
compared to naïve mice, 30.5 nmol/g vs 11.2 nmol/g. DISCUSSION
We synthesized a new stable Gd-DOTA based molecular probe that
contains a peroxidase reactive 5-hydroxytryptamide pendant arm. Like earlier
derivatives, in the presence of MPO and H2O2, the 5-hydroxytryptamide
moiety of Gd-5HT-DOTAGA is oxidized and radicalized, and undergoes a
polymerization reaction increasing its molecular weight and thus, its
relaxivity. Our results are in agreement with those previously reported for the
Gd-bis-5-HT-DTPA complex,2
that showed a 80% increase in r1
under the same reaction conditions as used here. We applied Gd-5HT-DOTAGA to a
model of bleomycin lung injury in which macrophage are recruited to the injury
site. Post probe T1-weighted UTE MR images of bleomycin injured mice show an “in situ” activation of the probe that
provides a site-specific signal enhancement by local accumulation in the
damaged tissue, and that is corroborated with the ex vivo Gd measurements.CONCLUSION
Gd-5-HT-DOTAGA is a new amplifiable
molecular probe that represents a potentially new way to assess pulmonary
inflammation by visualizing myeloperoxidase activity in damaged tissue.Acknowledgements
Work is supported by the NINDS grant NS091552 to A.B.References
1. Querol Sans, M.; Chen, J. W.; Weissleder, R.; Bogdanov, A. DTPA-bisamide-based MR Sensor Agents for Peroxidase Imaging. Org Lett (2005) 7, 1719-1722.
2. Chen, J. W.; Querol Sans, M.; Bogdanov, A.; Weissleder, R. Imaging of Myeloperoxidase in Mice by Using Novel Amplifiable Paramagnetic Substrates. Radiology (2006) 240, 473-481.
3. Levy, S. G.; Jacques, V.; Zhou, K. L.; Kalogeropoulos, S.; Schumacher, K.; Amedio, J. C.; Scherer, J. E.; Witowski, S. R.; Lombardy, R.; Koppetsch, K. Development of a Multigram Asymmetric Synthesis of 2-(R)-2-(4,7,10-Tris tert-Butylcarboxymethyl-1,4,7,10-tetraazacyclododec-1-yl)-pentanedioic Acid, 1-tert-Butyl Ester, (R)-tert-Bu4-DOTAGA. Organic Process Research & Development (2009) 13, 535-542.
Figures