3056
Facile and Novel Synthesis of MR/NIR Dual Modal Contrast Agent for In Vivo Non-invasive Molecular Imaging1Bioimaging Research Team, Korea Basic Science Institute, Cheongju-si, Korea, Republic of
Synopsis
We proposed the phase transferring method of hydrophobic magnetic nanoparticles without any chemical modifications, for use as a magnetic resonance (MR)/near-infrared (NIR) fluorescence bimodal imaging contrast agent. Indocyanine green (ICG) was used both as an optical component and a surfactant for phase transfer with no superfluous moiety. ICG-MNP-labeled dendritic cells presented MR/NIR dual-modal imaging properties with high and sensitive detection ability for a long time. We expect that this novel MR/NIR contrast agent with sensitive detection and simultaneous imaging capability can be used in the imaging and tracking of immune cells to confirm immunotherapeutic efficacy.
Purpose
The aim of this study was to propose a facile synthetic approach for the phase transfer of hydrophobic magnetic nanoparticles by the direct adherence of fluorescent probes, without any chemical modifications, for use as a magnetic resonance (MR)/near-infrared (NIR) fluorescence bimodal imaging contrast agent.Methods
Magnetic nanoparticles in organic solvent were added to an aqueous solution of ICG and emulsified using a microtip-probe sonicator. The organic solvent was evaporated by magnetic stirring until it was completely removed. The uncoated ICG molecules were removed by magnetic separation several times at 4°C. BMDCs were incubated with the ICG-MNPs for 24 hours and stimulated to maturation by LPS addition. The ICG-MNP-labeled or unlabeled cells (2 × 106) were injected into TNF-α-pretreated (24 hours before injection) mouse footpads. Axial images of the mouse popliteal or axillary lymph nodes were acquired using turbo-rapid acquisition with relaxation enhancement (Turbo-RARE) sequences with the following parameters: TR = 3,500 ms, TE = 36 ms, FOV = 30 × 30 mm2, slice thickness = 1 mm, matrix = 256 × 256, NEX = 4. The ICG-MNP-labeled DCs were injected into the mouse footpads. The mice were placed in a home-made high-quality in vivo imaging system. Fluorescence images of mice were acquired using an 808-nm diode laser.Results
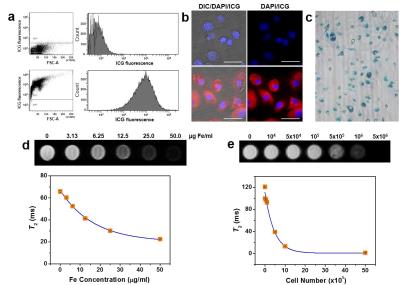
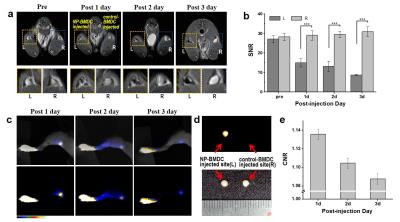
ICG coated MNPs (ICG-MNPs) were synthesized by a facile and novel phase transferring method. To conduct in vitro and in vivo imaging using ICG-MNPs, we first performed intracellular delivery of the particles into BMDCs (Figure 1). To measure the MR property of the labeled cells, T2-weighted MR images and T2 values were obtained as functions of treated concentrations and labeled cell numbers. The T2 value of the ICG-MNP-labeled DCs was only 10% of that of unlabeled DCs for 1 × 106 cells, indicating excellent MR imaging sensitivity. The fluorescence signals and Prussian blue (PB)-stained MNPs (Fe) were effectively detected in the cytosol for most cells. For in vivo MR and fluorescence imaging, C57BL/6 mice were subcutaneously injected at fore- or hind-leg footpads with the ICG-MNP-labeled or unlabeled DCs (Figure 2). After injection with 2 × 106 labeled (left pad) and unlabeled (right pad) DCs, MR and fluorescence images of popliteal lymph nodes were acquired for 3 days. T2-weighted MR images of the left popliteal lymph nodes after injection with the labeled DCs showed substantial darkening in the central zone of lymph nodes over time, whereas those of unlabeled DCs did not. These results indicate that ICG-MNP-labeled DCs migrated into lymph nodes from the injection site over several days after the injection and gradually accumulated inside the lymph node. Furthermore, fluorescence signals from the lymph nodes were observed for 3 days. Remarkably, even after 3 days after the injection, fluorescence signals could be detected at the lymph node.Discussion and conclusion
We designed a novel MR/NIR fluorescence imaging contrast agent by facile fluorescent dye-coating on MNPs. The fluorescent dye, ICG, was directly attached to the nanoparticle surface, enabling the nanoparticles to transition from a hydrophobic to a hydrophilic phase. ICG molecules were stably attached to MNPs. ICG-MNPs and ICG-MNP-labeled DCs presented MR/NIR dual-modal imaging properties with high and sensitive detection ability for a long time. Importantly, the migration of labeled DCs via lymphatic drainage and homing into lymph nodes was monitored by real-time MR/NIR imaging for 3 days. We expect that this novel MR/NIR contrast agent with sensitive detection and simultaneous imaging capability can be used in various fields, such as the imaging and tracking of immune cells to confirm immunotherapeutic efficacy. Furthermore, this principle can be also applied to the phase transfer of other kinds of hydrophobic particles using amphiphilic fluorescent dyes to develop multimodal probes for biomedical applications.Acknowledgements
This work was supported by a grant (K.S.H., D36401) from the Korea Basic Science Institute, the R&D Convergence Program (K.S.H., CRC-15-02-KRIBB), and the CAP (K.S.H., CAP-12-2-KBSI) funded by the Korea National Research Council of Science and Technology.References
1. Moon H, Park H E, Kang J, et al. Noninvasive Assessment of Myocardial Inflammation by Cardiovascular Magnetic Resonance in a Rat Model of Experimental Autoimmune Myocarditis. Circulation 2012;125(21):2603-2612.
2. Wu C, Xu Y, Yang L, et al. Negatively Charged Magnetite Nanoparticle Clusters as Efficient MRI Probes for Dendritic Cell Labeling and In Vivo Tracking. Adv Funct Mater 2015;25(23):3581-3591.
Figures