3055
Tumor-targeted alkylphosphocholine chelates for dual-modality PET/MR imaging1Department of Radiology, University of Wisconsin Madison, Madison, WI, United States, 2Department of Neurological Surgery, University of Wisconsin Madison, Madison, WI, United States, 3Department of Medicine - Endocrinology, University of Wisconsin Madison, Madison, WI, United States
Synopsis
Extensive structure-activity relationships studies have previously shown that alkylphosphocholine (APC) analogs selectively deliver radioiodine and larger fluorophores to a variety of tumor types in rodent models and humans1-3. To further explore the payload capacity of APCs, we synthesized several new APC-chelates and assessed their ability to deliver Gd (MRI) and 64Cu (PET) selectively to tumors in vivo. Prolonged T1-weighted signal enhancement following Gd-DOTA-APC injection was observed in all tumor models. Clinical PET/MR imaging of U87 flank xenograft at 24h and 48h post-administration of Gd-DOTA-APC and 64Cu-DOTA-APC demonstrated co-localization of T1-weighted signal enhancement and PET activity in the tumor.
Introduction:
We previously reported broad-spectrum cancer-specific radioiodinated APC uptake for PET based detection and targeted radiotherapy of both primary tumors and metastases in a wide variety of preclinical models and in human cancer patients1-3. If we are able to selectively target tumors in vivo, new APC-chelates would permit extension to a broad class of imaging and therapy metal ions.
The aims of this preliminary study were to assess the tumor targeting characteristics of Gd-DOTA-APC and 64Cu-DOTA-APC and their multimodality imaging potential through proof-of-concept PET/MR imaging of rodent models of human cancers. Concurrent work demonstrated excellent stability of the Gd chelate and relaxivities of this new T1-shortening macrocyclic contrast agent Gd-DOTA-APC at 1.5T and 3.0T. These preliminary findings demonstrate the broad-spectrum cancer-imaging and the PET/MR imaging potential of new APC chelates.
Methods:
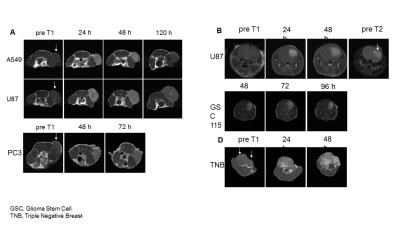
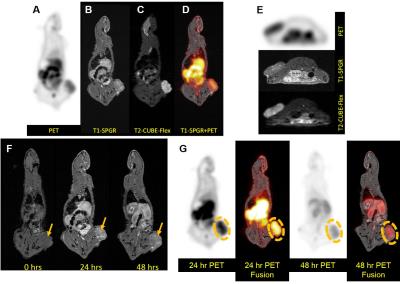
DOTA was conjugated to an APC analog using an amide linker, Gd or 64Cu was subsequently chelated onto DOTA-APC via standard methods. Gd-DOTA-APC was administered intravenously (approximately 0.12mg/kg) in 7 rodent models of human cancer, including two orthotopic glioblastoma models (U87 (n=2) and a patient-derived glioblastoma stem cell model GSC 115 (n=1) ), an αβ-overexpressing triple negative breast cancer model (n=4), a rat glioblastoma (U87) flank xenograft model (n=1) , a flank model of prostate cancer (PC3) (n=3), non-small cell lung cancer (A549) (n=3), and glioblastoma (U87) (n=3). In vivo imaging was performed on a 4.7T preclinical scanner (Agilent Technologies, Santa Clara, CA) pre-contrast and subsequently every day up to seven days after IV contrast injection. For simultaneous PET/MR of the rat flank, 64Cu-DOTA-APC (20mCi/kg) and Gd-DOTA-APC (0.12mg/kg) were mixed together and IV injected. In vivo PET/MR imaging was performed on a simultaneous 3T scanner (Signa PET/MR, GE Healthcare, Waukesha, WI) using the built-in posterior array coil. Sagittal 3D T1-weighted gradient echo (3.3ms TE, 7.7ms TR, 0.51x0.51x1mm pixel size, 244 Hz/pixel bandwidth, 15-degree flip angle, 4:53 scantime) and sagittal 3D CUBE-Flex (83.4ms TE, 2500ms TR, 100 ETL, 0.9x1.4x1mm pixel size, 326 Hz/pixel bandwidth, 5:41 scantime) images were obtained. A 20-minute PET acquisition was obtained simultaneously (1.17x1.17x2.78mm reconstructed pixel size, VPFX-S with 28/4 iterations/4 subsets) with MR. Ex vivo quantitative biodistribution studies were performed on a A549 flank xenograft mouse to determine the amount of Gd in different organs including the tumor at 72h post-injection and digested using a mixture of high-purity acids under microwave assistance. Gadolinium in the tissue digests are quantified using high-resolution (magnetic-sector) inductively-coupled plasma mass spectrometry (SF-ICPMS) (Wisconsin State Laboratory of Hygiene).Results:
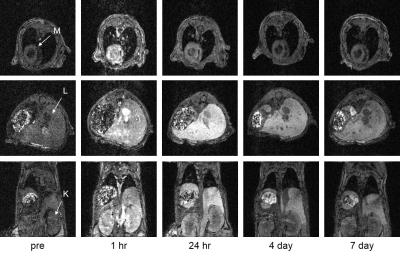
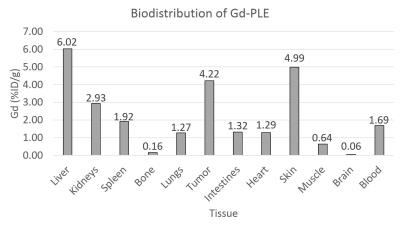
In vivo mouse imaging showed peak T1-weighted enhancement of the heart, liver, and kidneys 1 hour post-administration (Figure 1). Liver T1-weighted enhancement was the highest and most prolonged. Preliminary imaging studies performed in 6 murine animal models revealed prolonged tumor T1-weighted contrast enhancement for up to 120hr (Figure 2). Ex vivo tissue biodistribution from a A549 flank xenograft nude athymic mouse at 72h post contrast administration revealed high Gd concentration in the tumor (4.22% injected dose Gd/g tissue), and in organs of clearance - liver (6.02%ID/g), kidney (2.93%ID/g) (Figure 3). These organs constitute 3/4 organs of highest Gd concentration. Interestingly, skin uptake at 72h was also relatively high. Coadministration of 64Cu-DOTA-APC and Gd-DOTA-APC in a rat flank U87 xenograft model demonstrated co-localization of PET activity and MR enhancement in the tumor and organs of clearance at 24h and 48h post administration (Figure 4).Discussion:
These preliminary results demonstrate the broad cancer-targeting profile of this new DOTA-APC analog, and provide the first proof-of-concept potential of these APC chelates for targeted cancer imaging. The in vivo pharmacokinetics, biodistribution, and ex vivo biodistribution of Gd-DOTA-APC appear similar to those of a previous generation of iodine-labeled APC analogs3. Concurrent studies demonstrate striking longitudinal relaxivities at 1.5T and 3.0T that compare favorably to commercial agents, and excellent stability consistent with other macrocyclic agents. These data provide strong support for the translational use of DOTA-APC analogs for cancer-targeted MR imaging. The co-localization of 64Cu-DOTA-APC and Gd-DOTA-APC in a rat xenograft model illustrate the multi-modality imaging potential of this APC chelates4. Finally, this study shows that the tumor-targeting profile, biodistribution, uptake and pharmacokinetics are not reliant on drug dose as the MR contrast agent was administered at >1000x mass dose compared to the PET agent, which is ideal for a multimodality contrast platform agent. These results warrant further studies with other PET and therapeutic isotopes.Acknowledgements
We would like to thank the Deparment of Radiology and Department of Neurological Surgery at University of Wisconsin-Madison for the pilot funding to support this work.References
1 Pinchuk, A. N. et al. Synthesis and structure-activity relationship effects on the tumor avidity of radioiodinated phospholipid ether analogues. Journal of medicinal chemistry 49, 2155-2165 (2006).
2 Swanson, K. I. et al. Fluorescent cancer-selective alkylphosphocholine analogs for intraoperative glioma detection. Neurosurgery 76, 115-124 (2015).
3 Weichert, J. P. et al. Alkylphosphocholine analogs for broad-spectrum cancer imaging and therapy. Science translational medicine 6, 240ra275-240ra275 (2014).
4 Zhang, R. R., Swanson, K. I., Hall, L. T., Weichert, J. P. & Kuo, J. S. Diapeutic cancer-targeting alkylphosphocholine analogs may advance management of brain malignancies. CNS oncology 5, 223-231 (2016).
Figures