3053
A Janus Chelator Enables Biochemically Responsive MRI Contrast With Exceptional Dynamic Range1A. A. Martinos Center for Biomedical Imaging, MGH/ Harvard Medical School, Charlestown, MA, United States
Synopsis
Mn-JED is a new biochemically responsive MRI contrast agent that provides 9-fold relaxivity change by switching between the Mn(3+) and Mn(2+) oxidation states. The JED chelator is the only chelator that supports both the Mn(3+) and Mn(2+) oxidation states in biological milieu. Rapid interconversion between oxidation states is achieved by peroxidase activity (oxidation) and cysteine (reduction). Peroxidase activity is drastically elevated during acute inflammation. Thiols such as cysteine are overabundant in the microenvironment of proliferative tumors. Mn-JED provides a new paradigm for the design of biochemically responsive MRI contrast agents.
Purpose
Molecular MR imaging adds an aspect of biochemical specificity to the rich anatomic and physiologic information available through MRI. Gadolinium (Gd) based agents that undergo relaxivity change catalytically triggered by enzyme activity have been developed, but these agents suffer poor dynamic range for detection.1 The Gd-based agents that provide the largest relaxivity change do so at lower field strengths, ≤1.5T, and at ≥3T the dynamic range is impractically small.2 Manganese (Mn) complexes that undergo relaxivity change by switching between low-relaxivity Mn(3+) and high-relaxivity (Mn2+) oxidation states offers an interesting strategy to overcome the limitations of “activatable” Gd-based agents.3-5 Unfortunately, no Mn chelators support both oxidation states in biological milieu. The purpose of this study was to design a ligand to stably chelate both Mn(3+) and Mn(2+) in biological milieu, and to evaluate the relaxivity change achieved by biochemically mediated interconversion between the Mn oxidation states.Methods
Mn(3+)- and Mn(2+)-JED, Fig 1, were independently prepared, isolated, and characterized by high-pressure liquid chromatography (HPLC) and mass spectrometry (MS). Oxidation and reduction kinetics were measured using H2O2/ peroxidase and cysteine, respectively. The thermodynamic stability of Mn(3+)- and Mn(2+)-JED at pH 7.4 was determined by competition with ligands of known stability. T1-relaxivity in water and human blood plasma at 37 °C was determined from plots of 1/T1 vs. Mn concentration for at least 4 Mn concentrations, with T1 determined using an inversion recovery sequence. T1-weighted images were acquired at 25 °C, 4.7T using a 2D FLASH sequence, or a 2D FLASH sequence proceeded by an inversion pre-pulse to null signal from phantoms of a given T1 and maximizing contrast between adjacent phantoms.Results
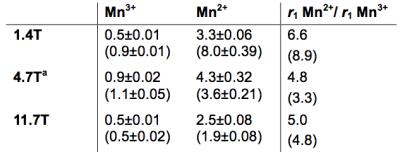
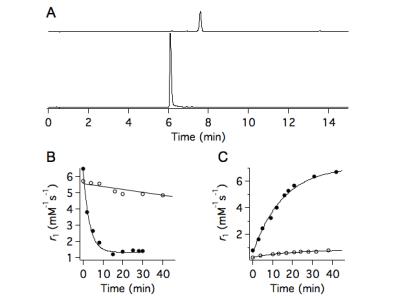
JED supports both Mn(3+) and Mn(2+) in water and blood plasma. Figure 2 shows relaxivity values at 1.4, 4.7, and 11.7T for Mn(3+)- and Mn(2+)-JED. The relaxivity of the Mn(2+) complex remains 3.3-5.0-fold greater than the Mn(3+) complex under all conditions measured. In human blood plasma at 1.4T, 37 °C, the Mn(2+) complex has 9-fold greater relaxivity than the Mn(3+) complex. Peroxidase-triggered Mn(2+) to Mn(3+) conversion occurs cleanly and without by-products. Figure 3A depicts HPLC traces of Mn(2+) and Mn(2+)-JED after incubation with H2O2/ peroxidase. The only species identified in the peroxidase incubated sample corresponds to Mn(3+)-JED. Fig 3B depicts the dynamic relaxivity change oxidation of Mn(2+)-JED by H2O2/ 15U/mL peroxidase, kobs = 19.1±4.8 s-1. Note that H2O2 alone triggers negligible conversion. The reaction is reversible. Mn(3+)-JED is rapidly reduced to Mn(2+)-JED in the presence of thiols, kobs = 3.6±0.5 s-1. Fig 3C depicts cysteine mediated reduction of Mn(3+)-JED in blood plasma triggered by addition of 5 mol. equiv. cysteine. No reduction occurs when incubating in blood plasma without added cysteine. The MRI contrast between equal concentration solutions of Mn(3+)- and Mn(2+)-JED is striking. Fig 4A shows a T1-weighted image at 4.7T of phantoms with 0.5 mM Mn(2+)-JED before and after peroxidase mediated oxidation. In this image, the Mn(2+) containing phantom is much brighter. Fig 4B depicts phantoms with 0.5 mM of the state of the art peroxidase sensing probe, Gd-bis-5HT-DTPA, that undergoes relaxivity change due to polymerization and subsequently restricted rotation of the Gd relaxation agent.6 At 4.7T, oxidation yields 15% relaxivity change – the difference is barely perceptible between the phantoms in a T1-weighted image. Figs4-D depict the same phantoms as 4A-B, respectively, but imaged using a 325 ms inversion pre-pulse to null the untreated phantom and generate positive contrast in the oxidized phantom. Regardless of the scanning protocol, the Mn-based agent senses peroxidase with superior dynamic range. Fig 4E compares percentage relaxivity change following oxidation of the Mn and Gd agents at 4.7. The Mn agent provides over an order of magnitude greater percentage relaxivity change compared to state of the art.Discussion
Mn-JED enables biochemically triggered interconversion between low relaxivity Mn(3+) and high relaxivity (Mn2+). Mn-JED is very sensitive to peroxidase activity with oxidation occurring in minutes in the presence of only 15 U/mL peroxidase, while peroxidase activities of 250,000 U/mL are reported in atherosclerotic lesions.7 We are unaware of any biochemically activated Gd-based agents that provide relaxivity change greater than Mn(3+) vs Mn(2+)-JED under any of the individual measurement conditions. At 4.7T, the biochemically mediated relaxivity change observed upon peroxidase oxidation of Mn is over an order of magnitude greater than the state of the art peroxidase-sensing Gd-based probe. Mn-JED offers a new paradigm for the development of biochemically responsive MRI contrast agents.Conclusions
Mn-JED enables biochemically-mediated relaxivity change with dynamic range that supersedes known biochemically activated Gd-based agents.Acknowledgements
This work was supported by grants from the National Heart, Lung, and Blood Institute (K25HL128899), the National Institute of Biomedical Imaging and Bioengineering (R01EB009062, R21EB022804) and instrumentation funded by the National Center for Research Resources and the Office of the Director (P41RR14075, S10RR023385, S10OD010650).References
1. Boros E, Gale EM and Caravan P. MR imaging probes: design and applications. Dalton Trans. 2015;44(11):4804-4818.
2. Caravan P, Farrar CT, Frullano L and Uppal R. Influence of molecular parameters and increasing magnetic field strength on relaxivity of gadolinium- and manganese-based T1-contrast agents. Contrast Media Mol Imag. 2009;4(2):89-100.
3. Gale EM, Mukherjee S, Liu C, Loving GS and Caravan P. Structure-redox-relaxivity relationships for redox responsive manganese-based magnetic resonance imaging probes. Inorg Chem. 2014;53(19):10748-10761.
4. Loving GS, Mukherjee S and Caravan P. Redox-Activated Manganese-Based Contrast Agent. J Am Chem Soc. 2013;135(12):4623.
5. Aime S, Botta M, Gianolio E and Terreno E. A p(O2)-Responsive MRI Contrast Agent Based on the Redox Switch of Manganese(II/III) Porphyrin Complexes. Angew Chem Int Ed. 2000;39(4):747-750.
6. Rodríguez E, Nilges M, Weissleder R and Chen JW. Activatable Magnetic Resonance Imaging Agents for Myeloperoxidase Sensing: Mechanism of Activation, Stability, and Toxicity. J Am Chem Soc. 2010;132(1):168-177.
7. Daugherty A, Dunn JL, Rateri DL and Heinecke JW. Myeloperoxidase, a Catalyst for Lipoprotein Oxidation, Is Expressed in Human Atherosclerotic Lesions. J Clin Invest. 1994;94(1):437-444.
Figures