3051
Cyclodextrin-based pseudo-rotaxanes as conjugatable molecular imaging biosensors for hyperpolarized 129Xe MRI1Lakehead University, Thunder Bay, ON, Canada, 2University of Guelph, 3University of Rhode Island, 4Lakehead University, MURILLO, ON, Canada
Synopsis
Hyperpolarized (HP) 129Xe molecular imaging technology has recently advanced in the detection of biochemically inactive supramolecular cage-molecules within a living mammalian model. Unfortunately, the natural bio-distribution of these biosensor molecules is non-specific, which makes it difficult to precisely localize them in vivo using HP 129Xe MRI. With the HyperCEST detection of easily conjugated cyclodextrin-based pseudo-rotaxanes, we have identified a critical advancement in 129Xe biosensor design by uncovering a novel biosensor, which has the potential to precisely detect markers of early disease in a human body with comparable sensitivity to PET but with the spatial resolution of MRI.
Audience and Purpose
Hyperpolarized (HP) 129Xe magnetic resonance (MR) imaging biosensors have the potential to be used as molecular imaging contrast agents similar to radioligands in positron emission tomography (PET). One of the difficulties in translating these imaging biosensors from in vitro demonstration to clinical use is synthesizing them in sufficient quantities and at a sufficient yield to be detectible within the human body 1. In this work, we have established a unique synthesis scheme in the development of a 129Xe imaging biosensor based on a γ-cyclodextrin (γ-CD) pseudo-rotaxane. MR detection is achieved via the HyperCEST effect and binding to a molecule of interest is provided by an affinity tag, easily conjugated to an alkyl chain, which threads the cavity of the γ-CD.Methods
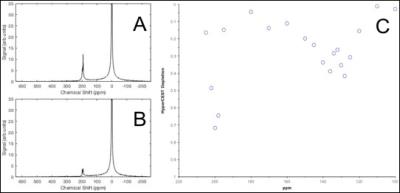
Naturally abundant 129Xe gas was polarized to 26-30% using a Xemed polarizer (Xemed, Durham, NH, USA). One mL of the pseudo-rotaxane sample (1.0 mM in PBS buffer) was placed into a glass-fritted phantom (Figure 1) which was positioned inside a custom RF coil tuned to the Larmor frequency of 129Xe at 3T (35.33 MHz). HP 129Xe gas was bubbled through the frit while NMR spectra were acquired using a Philips Achieva 3T clinical scanner. The RF pulse length was determined using the Ref B1, a parameter of Philips MR scanners. In this study, the B1 field strength was determined by the scanner to be 70 µT. Immediately prior to the acquisition of MR spectra, a pulsed saturation pre-pulse train consisting of 96- 20 ms 3-lobe sinc pulses with 0 ms pulse intervals was applied at various chemical shift frequency offsets. Free induction decay (FID) spectra were acquired for several scans at various frequency offsets, approximately 5 ppm apart. Each FID spectrum was acquired approximately 6 seconds apart. The mean signal-to-noise ratio (SNR) obtained from all control spectra for individual samples was used in the measurement of signal depletion. The SNR for each spectrum was calculated using MATLAB (MathWorks, Natick, MA, USA). To measure signal depletion, the mean HyperCEST (on-resonance) spectrum SNR was subtracted from the mean control (off-resonance) spectrum SNR. This difference was then divided by the mean off-resonance spectrum SNR to provide the signal depletion via HyperCEST.Results & Discussion
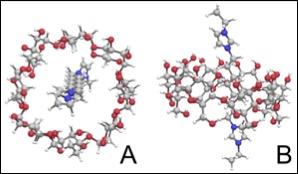
Pseudo-rotaxanes (1.0 mM) of α-, β-, and γ-CD threaded with a variety of lengths of ethylimidazolim alkane chains were tested. A maximum HyperCEST depletion of 32% for α-cyclodextrin pseudo-rotaxane, and 52% for γ-cyclodextrin pseudo-rotaxanes with 1,10-di(3-ethylimidazole) decane threaded through the cavity were observed (Figure 2). A HyperCEST depletion was not observable with the β-cyclodextrin pseudo-rotaxane. The maximum depletion for all cyclodextrin-based 129Xe biosensors occurred at approximately +127 ppm from the 129Xe gas phase signal. Cyclodextrins are somewhat tapered2, which makes them an ideal host for both HP 129Xe atoms and functionalized alkane chains (Figure 3). Cyclodextrin-based pseudo-rotaxanes are also appealing in HP 129Xe molecular imaging as they are easily synthesized and readily conjugated, both of which are critical for advancement in 129Xe biosensor technology. In conjunction with their MR detection via HyperCEST, cyclodextrin-based pseudo-rotaxanes have promising potential to specifically localize early disease markers at comparable sensitivity to radioligands in PET, however, without the effects of ionizing radiation and with the spatial resolution of MRI. Ultimately, the identification of cyclodextrin-based pseudo-rotaxanes as 129Xe biosensors marks an essential advancement in HP 129Xe molecular imaging which is necessary for the translation of 129Xe biosensor technology into a clinical imaging technique.Conclusions
This work is the first demonstration, to our knowledge, that identifies cyclodextrin-based pseudo-rotaxanes as suitable candidates for further investigation as molecular imaging agents for targeting areas of disease and imaging them with HP 129Xe MRI. Given the recent detection of a HyperCEST imaging biosensor within the body of a living mammalian model 3 we eagerly anticipate the in vivo demonstration of HyperCEST imaging cyclodextrin-based pseudo-rotaxanes to detect areas of pathology within living disease models.Acknowledgements
BP was supported by an Natural Sciences and Engineering Research Council (NSERC) undergraduate student research award. FH is supported by Canadian Institutes for Health Research (CIHR) and BrightFocus fellowships. MA is supported by an NSERC Discovery grant.References
1. Schroeder, L. In Hyperpolarized and inert gas MRI: From technology to application in research and medicine; Albert, M. S., Hane, F. T., Eds.; 2016.
2. Pinjari, R. V.; Khedkar, J. K.; Gejji, S. P. J. Incl. Phenom. Macrocycl. Chem. 2010, 66, 371.
3. Hane, F.T., Smylie, P.S., Li, T., Ruberto, J., Dowhos, K., Ball, I., Tomanek, B., DeBoef, B. and Albert, M.S. Contrast media mol. imaging. 2016
Figures