3045
Practical Considerations of Quantitative kPL Estimation in Hyperpolarized-13C Imaging in Response to Pulse Sequence Design and Parameters1Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2UCSF/UC Berkeley Graduate Program in Bioengineering, University of California, San Francisco, San Francisco, CA, United States, 3GE healthcare, Menlo Park, CA, United States
Synopsis
Hyperpolarized-13C MRI has recently enabled imaging of cancer pathophysiology with high spatiotemporal resolution in humans. Quantitative measure of tumor metabolism can be made possible by estimating conversion rate constants (e.g. kPL for pyruvate-to-lactate). We have identified 3 systematic sources affecting kPL estimation that were introduced by MR acquisition and pulse sequences – an RF-spoiling effect, a T2*-weighting factor, and a crusher flow-suppression phenomenon. These sources were investigated using a transgenic cancer model and simulations.
Purpose
Hyperpolarized-13C MRSI has enabled imaging of cancer pathophysiology with high spatiotemporal resolution in both human and translational settings1. Pyruvate-to-lactate conversion rate (kPL) is a quantitative measure of cancer metabolism and LDH activity. For quantitative analysis, it is important to identify systematic factors introduced by the MR acquisition. We investigated the impact of sequence design and parameters on estimation of kPL through imaging studies of transgenic mice with prostate cancer (TRAMP) using a 3D dynamic compressed-sensing 13C-EPSI (CS-EPSI) sequence recently applied for human imaging1 and simulations.Methods
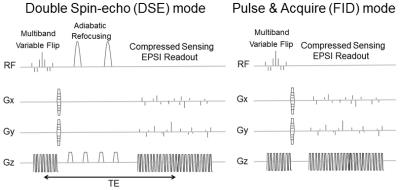
Sequences: A 3D dynamic CS-EPSI sequence provides 5-dimension (3 spatial, 1 temporal, 1 spectral) acquisition with high spatiotemporal resolution. Double spin-echo2 (DSE, TE=150ms) and FID (TE=6.3ms) acquisition modes (Fig.1A), as well as 2 modified sequence schemes (Figs.3A&4A) based on the FID mode were applied in HP-13C scans of TRAMP mice.
MRI experiments: Twelve TRAMP mice were studied using a clinical 3T scanner with a dual-tuned mouse coil for 13C and proton imaging. [1-13C]pyruvate was polarized using a preclinical HyperSense polarizer for 1.5 hours, reaching approximately 20-25% percentage polarization. Following dissolution, ~350ul of 80mM [1-13C] pyruvate was rapidly injected through a tail vein catheter over 15 seconds. Each 13C MR acquisition began at the end of injection.
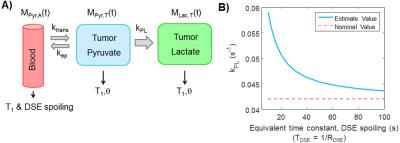
Simulations: A three-site compartmental model was applied to generate the simulated DSE-spoiled signal (Fig.2A). It consists of a vascular input (AIF) of pyruvate, as well as tumor pyruvate and lactate compartments3,4,5. The DSE spoiling was modeled as magnetization loss in the input function Mpyr,A(t) with equivalent spoiling rate RDSE. Metabolic conversion and signal loss mechanisms, such as RF excitations and T1 relaxation, were also included in the model. The kPL fit was estimated using the robust two-site exchange model also applied in vivo1,6. All simulations were performed on MATLAB.
Results and Discussions
One difference between DSE and FID mode, first observed by Josan7, is that the two adiabatic refocusing pulses may spoil the HP-13C spins near the coil edge, which predominantly affects the AIF of pyruvate. In simulations, RF spoiling on AIF was modeled as an equivalent spoiling factor RDSE. Increasing RDSE results in higher kPL estimates (Fig.2B) since the tumor pyruvate pool, directly supplied by AIF, decayed more rapidly than expected by the kinetic model.
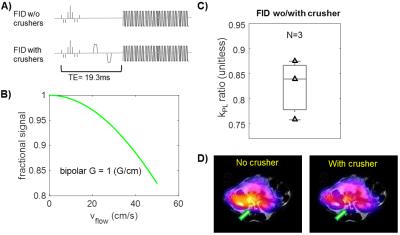
Secondly, the crusher gradients (G=1G/cm on x,y,z, δ = 2ms, Δ = 11.8ms, Venc = 0.21m/s, b = 0.02s/mm2) around the DSE refocusing pulses can act as flow-suppressing gradients, reducing vascular pyruvate signal and leading to a higher kPL estimate8, similarly to the DSE spoiling. Simulations yielded a ~10% loss for typical peak arterial blood velocity of 30(cm/s) in animals. Back-to-back HP-13C studies of TRAMP mice on the same day using FID mode scans with and without the crusher gradients demonstrated higher prostate tumor kPL estimates with the crusher (Fig.3C, kPL,crusher/kPL,w/o = 1.22±0.09, n = 3), corroborating the simulation results. Moreover, flow-suppression effects were preferentially seen near the major vasculature and renal arteries (Fig.3D).
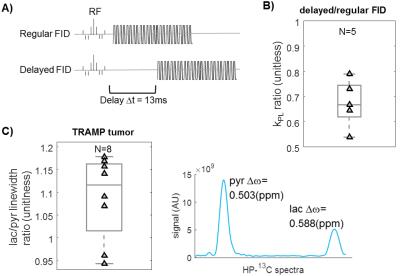
Finally, in vivo T2* is hypothesized to be much shorter in lactate than pyruvate due to J-coupling9,10. A “delayed” FID mode purposely introduced a longer echo time (Δtdelay=13ms) to introduce more T2* decay. As anticipated, lower tumor kPL was found using the delayed sequence vs. normal FID (Fig.4B, kPL,delay/kPL,FID = 0.67±0.09, n = 5), in back-to-back TRAMP tumor injections. Additionally, the lactate peak had broader linewidth than pyruvate in TRAMP tumor studies (Fig.4C), in agreement with the linewidth differences Marjańska9 and Chen11 observed due to lactate J-coupling that results in T2* weighting.
When comparing DSE and FID modes, a higher tumor kPL was observed using the DSE than FID mode (Fig.5A, kPL,FID/kPL,DSE = 0.48±0.20, N=7). This likely is a result of the DSE spoiling and crusher flow-suppression in the DSE sequence increasing the tumor pyruvate decay rate (Figs.2&3) and FID T2* weighting (Fig.4). It may also be a result of compartmental T2-weighting at the DSE TE=150ms, as pyruvate was measured to have a larger short-T2 component compared to lactate in TRAMP tumors10, which would further reduce the pyruvate signal in the DSE.
Conclusions
We have identified 3 systematic sources that can affect the quantitative kPL estimation using 3D dynamic EPSI sequence. These sources are particularly important in the context of clinical translation of HP-13C imaging, where use of spin-echoes is more challenging due to peak RF power and B1 homogeneity considerations. It is worth mentioning that while this study provides simulated and experimental estimates of these factors, the actual impact largely depends on the pathophysiology and pharmacokinetic behavior in vivo.Acknowledgements
The authors acknowledge funding from R01EB017449, R01EB013427, and P41EB013598.References
[1] Chen H-Y et al., “3D Dynamic Hyperpolarized 13C-Pyruvate MR Metabolic Imaging of Human Prostate Cancer”, ISMRM. 2016.
[2] Cunningham CH et al., “Double Spin Echo Sequence For Rapid Spectroscopic Imaging of Hyperpolarized 13C”, JMR. 2007. 187:357-362.
[3] von Morze C et al., “Imaging of Blood Flow Using Hyperpolarized [13C]Urea in Preclinical Cancer Models”, JMR. 2011. 33:692-697
[4] Kazan SM et al., “Kinetic Modeling of Hyperpolarized 13C Pyruvate Metabolism in Tumors Using a Measured Arterial Input Function”, MRM. 2013. 70:943–953
[5] Bankson JA et al., “Kinetic Modeling and Constrained Reconstruction of Hyperpolarized [1-13C]-Pyruvate Offers Improved Metabolic Imaging of Tumors”, Cancer Res. 2015. 75(22):4708-4717
[6] Bahrami N et al., “Kinetic and perfusion modeling of hyperpolarized 13C pyruvate and urea in cancer with arbitrary RF flip angles”, Quant Imaging Med Surg. 2014. 4(1):24–32
[7] Josan S et al., “Application of double spin echo spiral chemical shift imaging to rapid metabolic mapping of hyperpolarized [1 13C]-pyruvate”, JMR. 2011. 209:332–336
[8] Gordon JW et al., “Application of Flow Sensitive Gradients for Improved Measures of Metabolism Using Hyperpolarized 13C MRI”, MRM. 2016. 75:1242-1248
[9] Marjanska M et al., “In vivo 13C spectroscopy in the rat brain using hyperpolarized [1-13C]pyruvate and [2-13C]pyruvate”, JMR. 2010. 206(2):210–218
[10] Y-F Yen et al., “T2 relaxation times of 13C metabolites in a rat hepatocellular carcinoma model measured in vivo using 13C-MRS of hyperpolarized [1-13C]pyruvate”, NMR Biomed. 2010. 23(4):414–423
[11] Chen AP et al., “In vivo hyperpolarized 13C MR spectroscopic imaging with 1H decoupling”, JMR. 2009. 197:100–106
Figures