3043
A Preliminary Framework for Validation of HP MRI using Mass Spectrometry1Department of Imaging Physics, UT MD Anderson Cancer Center, Houston, TX, United States, 2Department of Head & Neck Surgery, UT MD Anderson Cancer Center, Houston, TX, United States, 3Department of Bioinformatics & Computational Biology, UT MD Anderson Cancer Center, Houston, TX, United States
Synopsis
Dynamic imaging of HP pyruvate shows tremendous promise for offering new insight into tumor metabolism with unprecedented sensitivity, specificity, and spatiotemporal resolution. Imaging constraints due to the finite, nonstationary, and non-renewable signal pool necessitate the use of complex imaging and reconstruction strategies, but current approaches to validation of complex HP MRI measurements are lacking and new methods are critically needed. In this work, we investigate a framework for external validation of quantitative HP MRI biomarkers of tumor metabolism using stable isotope tracer analysis (MS-SITA). We show good agreement between quantitative biomarkers of chemical conversion derived from HP MRI and MS-SITA.
Introduction
Dynamic spectroscopic imaging of hyperpolarized (HP) [1-13C]-pyruvate1 allows insight into tumor metabolism2,3 with unprecedented specificity and spatiotemporal resolution. Imaging of HP substrates such as pyruvate is challenging because signals are nonstationary, non-renewable, continually decaying due to excitation losses and T1 relaxation, and evolving in spatial, spectral, and temporal domains that must be appropriately encoded. Advanced imaging and data reduction strategies such as parallel imaging4, compressive sensing5, and model-based reconstruction6 have been used to improve spatiotemporal resolution without increasing the depletion of the HP signal pool through additional signal excitations. Although dynamic multispectral phantoms have been proposed7, additional methods to validate strategies for imaging and quantification of ephemeral HP substrates are critically needed. In this work, we describe a potential framework for validation of HP MRI using mass spectrometry stable isotope tracer analysis (MS-SITA).Methods
We recently introduced a suite of pharmacokinetic models to describe the evolution of HP pyruvate and lactate signals in vivo6. A similar model describes incorporation of a bolus of labeled pyruvate ($$$P_{M+3}^{0}$$$) into intracellular pools of pyruvate and lactate:
$$ \begin{bmatrix}P_{M+3}(t)\\ L_{M+3}(t)\end{bmatrix}= \frac{P_{M+3}^{0}}{{k_{PL}+k_{LP}}}\left ( \begin{bmatrix}k_{LP}\\ k_{PL}\end{bmatrix} + \begin{bmatrix}k_{PL}\\ -k_{PL}\end{bmatrix} e^{-(k_{PL}+k_{LP})t}\right ) \;\;\;\;\;\;[1] $$
If, after some time delay ($$$T_{d}$$$) metabolic activity and checmical exchange is halted, and mass spectrometry is used to determine the amount of labeled pyruvate ($$$P_{M+3}(T_{d})$$$), total pyruvate pool ($$$P_{\sum M}$$$), labeled lactate ($$$L_{M+3}(T_{d})$$$), and total lactate pool ($$$L_{\sum M}$$$) then Equation [1] can be rearranged to solve for $$$k_{PL}$$$. If reverse exchange ($$$k_{LP}$$$) can be ignored over this interval, then the expression simplifies to:
$$ k_{PL,MS}(T_{d})=\frac{1}{T_d}\ln \left ( 1+\frac{L_{M+3}(T_d)}{P_{M+3}(T_d)}\right ) \;\;\;\;\;\;[2] $$
To test the feasibility of this framework for comparison with $$$k_{PL}$$$, the apparent rate constant for conversion of HP pyruvate into lactate and quantitative imaging biomarker for tumor metabolism, we acquired paired HP MRS, DCE-MRI, and MS-SITA of animals bearing orthotopic anaplastic thyroid tumors. Animals were administered various doses of 2-deoxyglucose (2DG; 500 mg/kg IP 2h or 24+2h before scanning), then anesthetized using isoflurane and placed head-first and supine at isocenter of a 7T Biospec small animal imaging system (Bruker Biospin MRI). A dual-tuned volume resonator was used for 1H localizer scans and 13C signal excitation, and a 20mm surface coil (Rapid Biomedical) was placed over the tumor and used for 13C signal reception. Dynamic slice- and coil-localized pulse-acquire spectroscopy (TR=2s, FA=20°, SW=5kHz, NOP=2048, NR=96) was acquired beginning approximately 10s before 200uL injection of 80mM HP [1-13C]-pyruvate. HP MRS was then followed by DCE-MRI to assess the influence of perfusion and eliminate vascular parameters as unknowns in the pharmacokinetic analysis of HP signal evolution6. After imaging, tumors were surgically exposed and animals were administered 50uL of 160mM [U-13C6]-glucose. (Labeled glucose, rather than pyruvate, was used to eliminate intravascular concentration of the labeled substrate as a potential confound.) After 60-90s delay for label uptake ($$$T_d$$$), tumors were excised and quickly flash frozen, and stored at -80C until targeted tracer analysis was conducted using an Orbitrap Fusion Tribid mass spectrometer in our institutional Proteomics and Metabolomics Core Facility.
Results
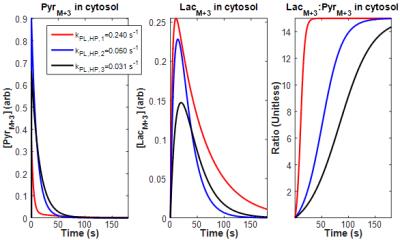
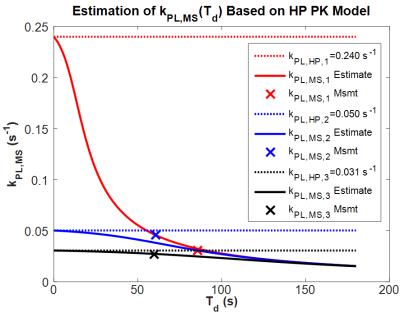
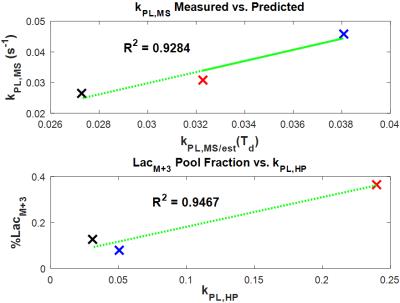
To estimate the temporal characteristics of incorporation of uniformly labeled glucose into pyruvate and lactate pools, $$$k_{PL}$$$ as measured from HP MRS was used in a version of Eq. [1] that was modified to account for vascular delivery of the labeled substrate in vivo (Figure 1). These plots suggest that pool ratios stabilize significantly faster than initially expected. Substituting these curves into Eq. [2], Figure 2 shows that $$$k_{PL,MS}$$$ is a far better estimate of $$$k_{PL}$$$ from HP MRS after short exposure times, and that accurate estimate of transient exchange kinetics become impossible once pool ratios equilibrate. Figure 3 shows strong correlation between $$$k_{PL,MS}$$$ calculated from MS-SITA using Eq. [2] and $$$k_{PL,MS/est}$$$ that was calculated by inserting $$$k_{PL}$$$ from HP MRS into Eq. [1] and Eq. [2] at a matched exposure time for each tumor. Figure 3 also shows good correlation between $$$k_{PL}$$$ from HP MRS and the labeled fraction of the lactate pool.Discussion
The framework described above allows comparison of the rapid and transient kinetics of labeled metabolic agents over a similar time scale and using complimentary technologies. Good agreement between measures of $$$k_{PL}$$$ as derived from HP MR and MS-SITA suggest this framework as a promising method for quantitative external validation of metabolic imaging biomarkers derived from HP MR. More data is needed in order to demonstrate accuracy of this model both at lower $$$T_d$$$ and after pool fractions equilibrate.Acknowledgements
This work was supported in part by the by the National Institutes of Health (P30-CA016672; R21-CA178450) and the Cancer Prevention & Research Institute of Texas (RP140021-P5).References
1. Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci USA 100(18):10158-63, 2003.
2. Golman K, in 't Zandt R, Thaning M. Real-time metabolic imaging. Proc Natl Acad Sci U S A 103(30):11270-5, 2006.
3. Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, Reed G, Carvajal L, Small EJ, Munster P, Weinberg VK, Ardenkjaer-Larsen JH, Chen AP, Hurd RE, Odegardstuen LI, Robb FJ, Tropp J, Murray JA. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Sci Transl Med 5(198):198ra08, 2013.
4. Ohliger MA, Larson PE, Bok RA, Shin P, Hu S, Tropp J, Robb F, Carvajal L, Nelson SJ, Kurhanewicz J, Vigneron DB. Combined parallel and partial fourier MR reconstruction for accelerated 8-channel hyperpolarized carbon-13 in vivo magnetic resonance Spectroscopic imaging (MRSI). J Magn Reson Imaging 38(3):701-13, 2013.
5. Larson PE, Hu S, Lustig M, Kerr AB, Nelson SJ, Kurhanewicz J, Pauly JM, Vigneron DB. Fast dynamic 3D MR spectroscopic imaging with compressed sensing and multiband excitation pulses for hyperpolarized 13C studies. Magn Reson Med 65(3):610-9, 2011.
6. Bankson JA, Walker CM, Ramirez MS, Stefan W, Fuentes D, Merritt ME, Lee J, Sandulache VC, Chen Y, Phan L, Chou PC, Rao A, Yeung SC, Lee MH, Schellingerhout D, Conrad CA, Malloy C, Sherry AD, Lai SY, Hazle JD. Kinetic modeling and constrained reconstruction of hyperpolarized [1-13C]-pyruvate offers improved metabolic imaging of tumors. Cancer Res 75(22):4708-17, 2015.
7. Walker CM, Lee J, Ramirez MS, Schellingerhout D, Millward S, Bankson JA. A catalyzing phantom for reproducible dynamic conversion of hyperpolarized [1-¹³C]-pyruvate. PLoS One 8(8):e71274, 2013.
Figures