3042
Characterization and flip angle calibration of 13C surface coils for hyperpolarization studies1Department of Electrical Engineering, Technical University of Denmark, Kgs. Lyngby, Denmark, 2Department of Clinical Physiology, Nuclear Medicine & PET and Cluster for Molecular Imaging, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark, 3Department of Veterinary Clinical and Animal Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Frederiksberg C, Denmark
Synopsis
The aim of the present work is to address the challenge of optimal flip angle calibration of 13C surface coils in hyperpolarization studies. To this end, we characterize the spatial profile of the flip angle and demonstrate that it allows for a simple calibration improving the signal-to-noise ratio for hyperpolarized 13C magnetic resonance spectroscopic imaging.
Introduction
For hyperpolarized 13C magnetic resonance spectroscopic imaging (13C-MRSI), flip angle (FA) calibration is challenged. Therefore, a 13C enriched phantom placed outside the imaged object is commonly used as reference. This does, however, give potential errors in case of RF transmit field (B1) inhomogeneities directly affecting the FA. The common use of single-channel surface coils with strong RF transmit profiles for 13C-MRSI enhances the importance of addressing FA calibration.
The aim is to address optimal FA calibration of 13C surface coils. We characterize the spatial profile of the FA and demonstrate that it allows for a simple calibration improving the SNR.
Methods
Theory: The B1 profile in the axial-axis of a surface loop coil is given by the Biot-Savart law. The coil transmitter voltage $$$V$$$ required for constant FA as function of distance is:
$$V(z)=c\cdot\left(z^2+a^2\right)^{3/2}\quad\quad\text{(1)}$$
where $$$c$$$ is an acquisition- and coil-dependent constant, $$$z$$$ is the axial-axis distance, and $$$a$$$ is the radius of the coil loop.
From Eq. (1) the following expression can be stated:
$$V_{90}(z)=V_{90,REF}\cdot\left(\frac{z^2+a^2}{z^2_{REF}+a^2}\right)^{3/2}\quad\quad\text{(2)}$$
Using Eq. (2), with a 90° FA calibrated at a reference position through determination of $$$V_{90,REF}$$$, a calibration $$$V_{90}(z)$$$ can hereby be obtained at an arbitrary position $$$z$$$ on the axial-axis.
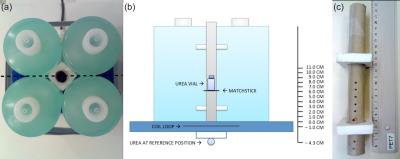
Phantom experiments: To verify Eq. (2) the FA profile was characterized for three different RF surface coils using the phantom setup in Figure 1.
The coils were: 1) a dual-tuned, 1H/13C, transmit/receive 11 cm flex coil, 2) a 13C, transmit/receive 12 cm loop coil, and 3) a 13C, transmit/receive 20 cm loop coil (RAPID Biomedical). A 5.5 mL vial of 4.0 M 13C-urea was used for FA calibration. All scans were performed on the integrated 3T PET/MRI system (Siemens Biograph mMR).
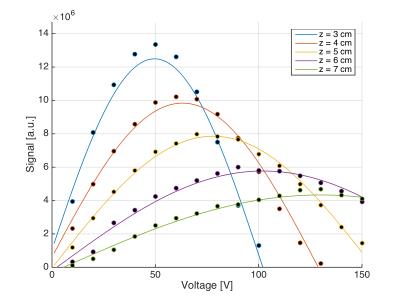
The vial was placed at a new position for each scan session, consisting of a localizer followed by FA calibration. For calibration a series of FID measurements with increasing transmitter voltage (10 V steps) were acquired [TR/TE 10,000/0.35 ms, FA 90°, BW 3,000 Hz, transmitter voltage 10 to 300 V (loop coils), transmitter voltage 10 to 150 V (flex coil)]. Spectra were centered on 13C-urea. A sinusoidal fit of the 13C-urea peak versus transmitter voltage was used to determine $$$V_{90}$$$. Eq. (1) was fitted to the calibration $$$V_{90}$$$ versus position of the 13C-urea vial. Experiments were repeated at different days and with different coil loads.
In vivo experiment: The feasibility of the calibration method was demonstrated in a canine cancer patient (39 kg) with a biopsy-verified axillar soft tissue sarcoma scanned as part of clinical staging work up. Patient handling and acquisition details are given in Gutte et al. 20151.
Hyperpolarized 13C-CSI was acquired with $$$V_{90,REF}=87.4$$$ V as measured using the calibration phantom positioned at the coil surface (equivalent to 90° at 2.3 cm). The CSI slice (13 mm slice thickness) was aligned parallel to the flex coil at a depth of 4 cm. To compensate for the FA loss a second CSI was obtained with $$$V_{90}=136$$$ V calculated by means of Eq. (2).
Voxel-wise SNR was estimated as the total carbon signal (sum of absolute peak values for pyruvate, pyruvate-hydrate, lactate, alanine, and bicarbonate) divided by five times the spectral noise (standard deviation of the real spectrum in noise regions).
Results
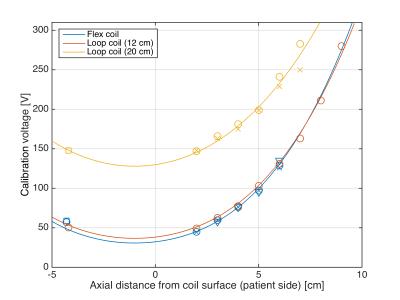
Figure 2 shows an example of FA calibration measurements and sinusoidal fits at different positions of the urea vial. The resulting calibration voltage $$$V_{90}$$$ and fits to Eq. (2) are shown in Figure 3. The fitting coefficients were: flex coil, $$$c=0.1847$$$ V/cm3 ($$$r^2=0.9807$$$); loop coil (12 cm), $$$c=0.1697$$$ V/cm3 ($$$r^2=0.9959$$$); loop coil (20 cm), $$$c=0.1280$$$ V/cm3 ($$$r^2=0.9681$$$). As can be appreciated in Figure 3 and by the $$$r^2$$$-values, data obtained for different days and loads follow the same curve.
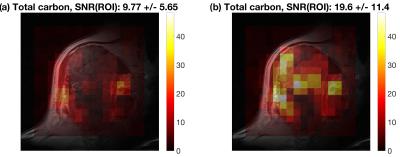
Figure 4 shows SNR for the two 13C-MRSI scans of the soft tissue sarcoma. The SNR improvement factor was 2.01. The noise level was constant.
Discussion
The study demonstrates feasibility of a FA calibration method for hyperpolarization studies using surface coils. Phantom experiments showed robustness of the method. Feasibility of implementation is shown in the presented canine cancer patient. However, the SNR improvement estimated to a factor 2.01 is larger than the increase in FA voltage (a factor 1.56). Therefore, the signal changes between scans cannot solely be attributed to change in FA. Tissue perfusion changes during anesthesia could also play a role.Conclusion
The FA profile of a surface loop coil can be utilized to improve SNR for hyperpolarized 13C-MRSI studies. Simple implementation and feasibility was demonstrated in a hyperpolarized 13C-MRSI canine cancer patient.Acknowledgements
No acknowledgement found.References
1. Gutte H, Hansen AE, Larsen MM, et al. Simultaneous Hyperpolarized 13C-Pyruvate MRI and 18F-FDG PET (HyperPET) in 10 Dogs with Cancer. J Nucl Med. 2015;56(11):1786-1792.Figures